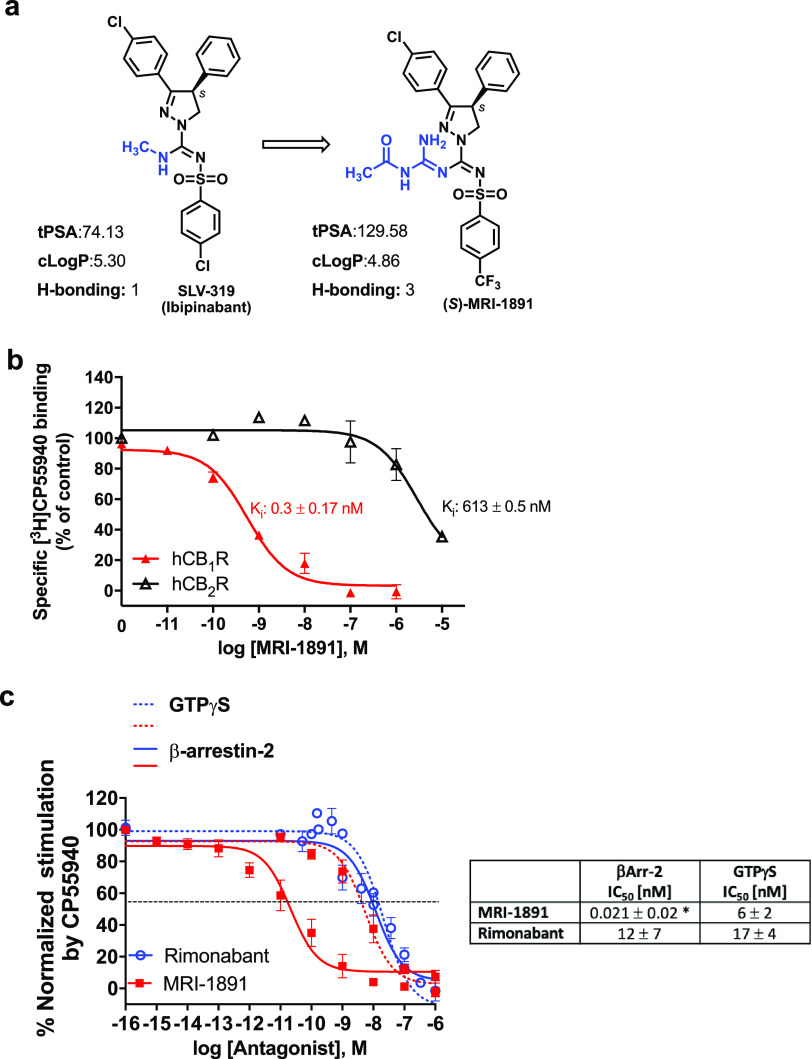
Figure 1.
(a) Chemical structure and physicochemical properties of (S)-MRI-1891 and its brain-penetrant parent compound SLV-319 (ibipinabant); (b) binding affinity of (S)-MRI-1891 to human CB1R and CB2R as determined by displacement of a radiolabeled cannabinoid agonist and crude membrane preparations from CHO-K1 cells stably transfected with hCB1R or hCB2R, as described in the Supporting Information, n = 3. (c) Inhibition of CB1R-agonist-induced GTPγS binding (dotted lines) and β-arrestin-2 recruitment (solid lines) by (S)-MRI-1891 (red) or rimonabant (blue), using hCB1R-CHO-K1 cell membrane (PerkinElmer, ES-110-M400UA) and PathHunter eXpress CNR1 CHO-K1 β-arrestin-2 assay, 93–0959E2CP0M, as described in the Supporting Information. Values represent mean ± SEM from 3–6 independent experiments. *, significant difference (P < 0.05) from IC50 values for inhibiting hCB1R-GTPγS signaling, as determined by t-test.