To the Editor:
Erythrocytosis is diagnosed when the hemoglobin (Hb) and/or hematocrit (Hct) is above the normal range for the subject age and sex.1 The number and order of tests to investigate an absolute erythrocytosis may vary in different centers. Patients who do not have any form of known primary or secondary erythrocytosis (SE) are diagnosed as idiopathic erythrocytosis (IE).2 While polycythemia vera (PV) has a wellknown and evidence-based treatment algorithm, which involves the use of low-dose aspirin and phlebotomy to keep the Hct below the target of 45% in all patients, and additional cytoreduction for high risk patients according to their age and/or previous thrombotic event,3 there is no consensus regarding the best way to manage patients with SE or IE. Although guidelines for SE from the British Society for Hematology suggest that phlebotomy should be considered in patients with a recent thrombotic event, additional risk factors for thrombosis, or Hct higher than 56%,3 we acknowledge that they are not widely accepted or used, and treatment decisions, either in terms of antiplatelet agents or phlebotomy, are rather made on a case by case basis, after an individualized risk-benefit assessment.
We performed a study to survey hematologists who regularly treat patients with myeloproliferative neoplasms (MPNs) regarding their standard practice when dealing with erythrocytosis. An online survey (surveyMonkey) was distributed by email to clinical hematologists practicing in Spain, Israel, United States, Italy, United Kingdom, Austria, and Ireland. The survey questions are shown in Supplementary Material.
One hundred and thirty-four physicians responded to the survey, including 35 physicians from Spain, 32 from Israel, 26 from the United States, 23 from Italy, 13 from the United Kingdom, and 5 from other countries (2 from Ireland and 3 from Austria).
First, we focused on the criteria used to define erythrocytosis and the tests performed to identify the cause of erythrocytosis. There is a general agreement (101 out of 134 physicians, 75.4%) in the use of lower thresholds for Hb and Hct (>165 g/L or 49% in men and >160 g/L or 48% in women) to diagnose erythrocytosis, according to the new 2016 WHO criteria.4 A small subgroup of 19 physicians (14.2%), mainly from the United Kingdom and Ireland (9 and 2 physicians, respectively), use the BSH criteria (Hct > 52% in men and >48% in women).5 Most of physicians do not have access to red cell mass evaluation (RCM) (95 of 134, 70.9%). Only four of those who have access to RCM study (10.4%) routinely use this diagnostic test. The access to RCM is surprisingly different among countries (P < .001), being available for more than half of physicians only in the United Kingdom (69.2%) and Spain (57.1%) compared to 15.4% of physicians in the United States, 13% in Italy, and 6.3% in Israel.
There is a good agreement in the use of bone marrow biopsy (BMB) in the diagnostic work-up of a patient with a suspect of MPN. Half of physicians perform BMB in all cases on suspection of MPN, a quarter of physicians perform BMB only in cases with suspected ET or PMF. The use of BMB significantly differs across countries (P < .001), being performed by 91.3% of physicians in Italy, 61.5% in the United States, 37.1% in Spain, 28.1% in Israel, and 15.4% in the United Kingdom.
The first-line assessments shared by at least half of hematologists include full blood count, serum erythropoietin, JAK2 V617F mutation, arterial oxygen saturation, serum ferritin, and renal and liver function tests. Responses regarding second-line investigations are more heterogenous, with only exon 12 JAK2 mutations, BMB and sleep study performed by more than 50% of physicians.
The term “idiopathic erythrocytosis” is used by most of physicians, mainly when all other causes of erythrocytosis are excluded.
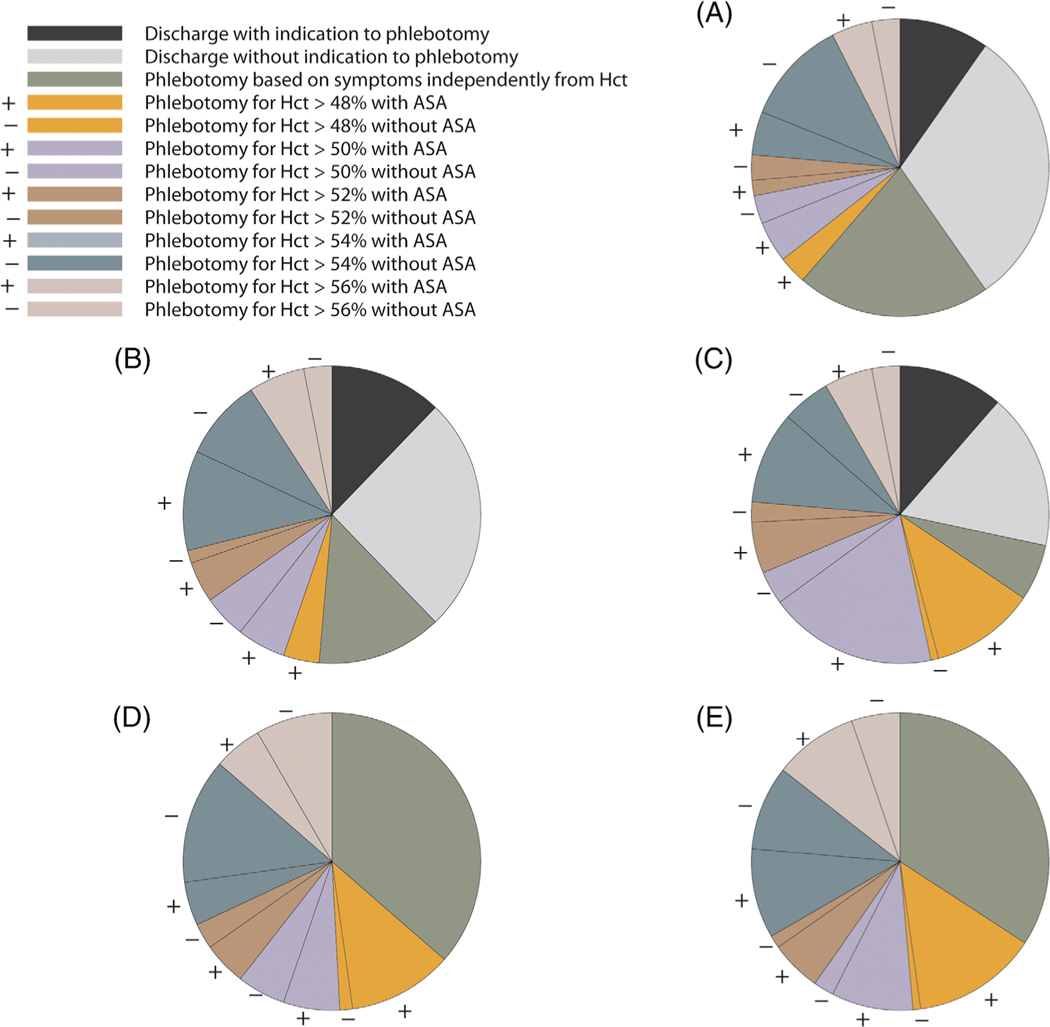
As second point, we focused on the treatment used in five common scenarios of SE or IE, as reported in Figure 1. The two groups of scenarios (SE in case A, case B, case C; IE in case D and case E) has been conceived with a progressive increase of vascular risk factors to check whether these risk factors could influence the treatment choice. We observed a marked heterogeneity in treatment practice, regarding both the Hct threshold (ranging from 48% to 56%) and the use of antiplatelet agents. The frequency of phlebotomy only in case of symptoms related to hyperviscosity decreases when moving from isolated erythrocytosis (scenario A) to erythrocytosis combined with cardiovascular risk factors (scenario B), and erythrocytosis combined with cardiovascular risk factors and previous thrombosis (scenario C). Moreover, the frequency of the more intervental approach (phlebotomy to keep Hct below 48% combined with antiplatelet) increases moving from scenario A to B to C. Also the use of antiplatelet agents increases moving from scenario A to B to C. This suggests a general feeling that the clinical context (risk factors, previous history) could directly affect the thrombotic risk, irrespectively of the elevated Hct, as recently reported by Gordeuk et al.6 This is in contrast with the clinical practice in PV where the Hct target (below 45%) is defined independently from the coexisting risk factors.
FIGURE 1.
Physicians’ treatment decisions for each of the five clinical scenarios. A, A 50-year-old man who presents with smoke-related, secondary erythrocytosis, but who lacks any other cardiovascular risk factors; B, A 50-year-old man, who presents with smoke-related, secondary erythrocytosis and concomitant hypertension; C, A 50-year-old man, who presents with smoke-related, secondary erythrocytosis, and a previous deep venous thrombosis; D, A 50-year-old man, who presents with idiopathic erythrocytosis without any other cardiovascular risk factors; E, A 50-year-old man, who presents with idiopathic erythrocytosis and concomitant hypertension. The association or not of antiplatelet agents was represented as +/− for each Hct threshold. ASA, antiplatelet agents; Hct, hematocrit
Patients with IE continue to be followed up, albeit with different timelines (every 6 months by 37.9% of physicians, every 12 months by 6.8% of physicians, according to Hct levels by 45.5% of physicians and according to symptoms independently from Hct by 9.8% of physicians), suggesting a common perception of this condition as a risky situation potentially evolving to PV or an unrecognized PV.
In conclusion, we observed a good agreement among hematologists regarding the diagnostic procedures and a widespread awareness regarding the importance of the new 2016 WHO criteria and bone marrow histology. On the other side, we observed a marked heterogeneity in treatment practice, regarding both the Hct threshold and the use of antiplatelet agents. A prospective study enrolling patients with secondary or idiopathic erythrocytosis would be useful to assess the real thrombotic risk of these patients during follow-up and the real protective effect of phlebotomy and antiplatelet agents to prevent the vascular risk.
Supplementary Material
ACKNOWLEDGMENTS
The study was supported by Fondo per il Finanziamento delle Attività Base di Ricerca (FFABR) and by a grant from Italian Ministry of Health for young researchers (GR-2016–02361272) to ER. The authors wish to acknowledge all their colleagues who contributed to this study by completing the online survey.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
CONFLICT OF INTEREST
Nothing to disclose.
References
- 1.McMullin MF. Investigation and management of erythrocytosis. Curr Hematol Malig Rep. 2016;11(5):342–347. [DOI] [PubMed] [Google Scholar]
- 2.McMullin MF. Idiopathic erythrocytosis: a disappearing entity. Hematology am Soc Hematol Educ Program. 2009;2009:629–635. [DOI] [PubMed] [Google Scholar]
- 3.McMullin MFF, Mead AJ, Ali S, et al. A guideline for the management of specific situations in polycythaemia vera and secondary erythrocytosis: a British Society for Haematology Guideline. Br J Haematol. 2019;184(2): 161–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. [DOI] [PubMed] [Google Scholar]
- 5.McMullin MF, Reilly JT, Campbell P, et al. Amendment to the guideline for diagnosis and investigation of polycythaemia/erythrocytosis. Br J Haematol. 2007;138(6):821–822. [DOI] [PubMed] [Google Scholar]
- 6.Gordeuk VR, Key NS, Prchal JT. Re-evaluation of hematocrit as a determinant of thrombotic risk in erythrocytosis. Haematologica. 2019; 104(4):653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.