Abstract
Background & Aims:
Bariatric surgery is common, but alcohol misuse has been reported following these procedures. We aimed to determine if bariatric surgery is associated with increased risk of alcohol-related cirrhosis (AC) and alcohol misuse.
Methods:
Retrospective observational analysis of obese adults with employer-sponsored insurance administrative claims from 2008–2016. Subjects with diagnosis codes for bariatric surgery were included. Primary outcome was risk of AC. Secondary outcome was risk of alcohol misuse. Bariatric surgery was divided into before 2008 and after 2008 to account for patients who had a procedure during the study period. Cox proportional hazard regression models using age as the time variable were used with interaction analyses for bariatric surgery and gender.
Results:
“194,130 had surgery from 2008–2016 while 209,090 patients had bariatric surgery prior to 2008. Age was 44.1 years, 61% women, and enrollment was 3.7 years. 4,774 (0.07%) had AC. Overall risk of AC was lower for those who received sleeve gastrectomy and laparoscopic banding during the study period (HR 0.4, p<0.001; HR 0.43, p=0.02) and alcohol misuse increased for Roux-en-Y and sleeve gastrectomy recipients (HR 1.86 and 1.35, p<0.001, respectively). In those who had surgery before 2008, women had increased risk of AC and alcohol misuse compared to women without bariatric surgery (HR 2.1 (95% CI: 1.79–2.41) for AC; HR 1.98 (95% CI 1.93–2.04)).”
Conclusions:
Bariatric surgery is associated with a short-term decreased risk of AC but potential long-term increased risk of AC in women. Post-operative alcohol surveillance is necessary to reduce this risk.
Keywords: alcohol-related liver disease, alcohol dependence, gender, obesity
Lay Summary
Weight loss surgery is effective for obesity treatment but has been shown to result in increased alcohol misuse post-operatively which could increase the risk of alcohol-related liver disease. We examined the risk of alcohol-related cirrhosis and alcohol misuse in a cohort of patients with private insurance and found that the short-term risk of alcohol-related cirrhosis after bariatric surgery was reduced, but the risk of alcohol misuse was increased. In the long-term, we found that the risk of alcohol-related cirrhosis was increased, especially in women.
Introduction
The obesity epidemic in the United States (US) has increased demand for weight loss solutions. As a result, bariatric surgery has emerged as one of the most common surgical procedures performed for treatment of obesity. In the US, sleeve gastrectomy and Roux-en-Y gastric bypass are the most common procedures performed, with adjustable gastric banding being less prevalent and declining (1). Long-term outcomes typically indicate durable weight loss and improvement in quality of life as well as many metabolic risk factors, such as diabetes and hypertension 1. While many patients undergoing bariatric surgery have fatty liver, including non-alcohol-related steatohepatitis (NASH), those who lose 10% or more of their body weight following gastric bypass may see substantial decreases in not only steatosis but also fibrosis which typically would be expected to protect bariatric surgery patients from cirrhosis, at least in the short term 2.
While obesity-related complications appear to improve over time, hazardous alcohol misuse both before and after bariatric surgery is a growing clinical concern leading to recommendations for preoperative screening for alcohol misuse3. Despite these efforts to screen out patients with heavy alcohol misuse prior to surgery, multiple studies document postoperative substance use and alcohol misuse disorder rates ranging from 7% to 33%4,5. The effect of bariatric surgery on the subsequent risk of alcohol-related cirrhosis (AC), however, is unclear, as there are few well-characterized, longitudinal prospective cohorts of bariatric surgery with enough follow-up time to allow for cirrhosis development. A key mediating factor may be alterations in alcohol metabolism following both Roux-en-Y gastric bypass and sleeve gastrectomy, whereby equivalent doses of alcohol result in higher peak blood alcohol levels, more prolonged alcohol elimination time, and greater levels of subjective intoxication6,7. Bypass of gastric alcohol dehydrogenase has been hypothesized as a potential mechanism for these observed differences.
More women than men undergo bariatric procedures8, and gender also plays an important and multifactorial role in the risk of developing AC. Women are more susceptible to liver disease at lower doses of alcohol, a poorly understood phenomenon that is likely related to differential distribution of hepatic alcohol dehydrogenase, differences in body composition, or to hormonal differences between sexes9. Women also may have some unique aspects to alcohol misuse disorders (AUD), which often facilitate the development of AC, including different AUD symptom presentation and course and suboptimal diagnosis, screening, and intervention in women compared to men10,11. Due to this confluence of risk factors, we sought to determine if bariatric surgery increases the risk of AC and whether the effect is more pronounced in women.
Methods
Population
We used the Marketscan Commercial Claims and Encounters dataset from 2008–2016 for our analysis. Marketscan is a large, nationally-representative administrative claims dataset composed of over 100 million unique enrollees with patient-specific data on inpatient, outpatient, facility, and pharmaceutical claims. The structure of the dataset allows a single patient with continuous coverage to be followed across multiple years of enrollment in both inpatient and outpatient settings. It is one of the largest and most comprehensive claims datasets and has been widely used in healthcare studies, including studies of alcohol-related liver disease and AC 12–15. Marketscan’s enrollment nearly approximates the entire population with employer-sponsored insurance (ESI) in the US which, in 2012, included 115,510,639 adults ages 18–64. All data were weighted to reflect the full Marketscan population.
Inclusion & Exclusion Criteria
We included all adult subjects, ages 18–64, in Marketscan who had at least one calendar year (360 days) of continuous coverage, which included the index cirrhosis diagnosis and any bariatric surgical procedure codes (see Figure 1). We sought to compare outcomes for those who received bariatric surgery compared to those who were eligible but did not receive surgery; therefore we restricted our initial cohort to only obese subjects. We included all subjects with an obesity code (see Supplemental Information), as these would be the only patients eligible for bariatric surgery, and, from this population of obese subjects, then retained all enrollees with bariatric surgery and all enrollees with cirrhosis. We excluded patients with hepatitis C from the primary analysis, but performed a sensitivity analysis in these patients. Patients who had bariatric surgery after a cirrhosis code was present (n=1803) were excluded from primary outcome analysis but retained for secondary outcome analysis (alcohol misuse).
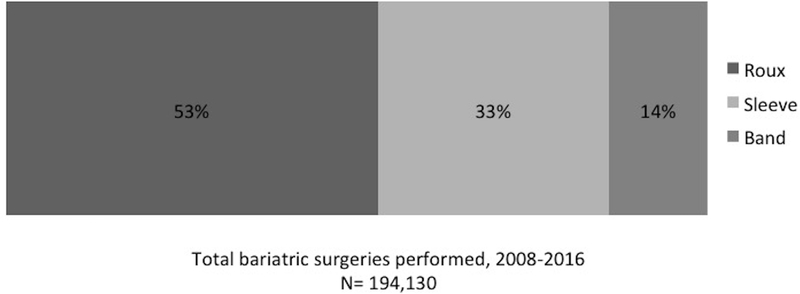
Figure 1.
Types of bariatric surgery performed during the study period (2008–2016) (n=194,130).
Outcomes and Variables
Our primary outcome was risk of AC after bariatric surgery, defined as the hazard ratio of a new AC diagnosis. Our secondary outcome was risk of clinically relevant and diagnosed alcohol misuse after bariatric surgery, again expressed as a hazard ratio. ICD-9 and ICD-10 codes were used to define bariatric surgical procedures as well as the primary and secondary outcomes of alcohol-related cirrhosis and alcohol misuse (see Supplemental Information). The structure of the MarketScan database allows for determination of procedures performed during the study period through the use of specific procedure codes, indicating that the subject had surgery on that date. However, based on diagnosis codes including in the Supplemental Information, many patients (n=209,090) had bariatric surgery prior to 2008 when our study period begins. These patients were included due to concern that alcohol misuse after bariatric surgery may take years to result in AC; therefore inclusion of only patients with incident surgeries during 2008–2016 would bias results and given an inaccurate picture of the short- and long-term potential risk of AC. These surgeries are captured with non-procedural diagnosis codes (for example, Z98.84 Bariatric surgery status (includes history of bariatric surgery); see Supplemental Information for more codes).
Bariatric surgery was therefore defined as follows: Patients who had a procedure code for a bariatric surgery, indicating that they had had a surgical procedure on that date during the study period, were defined as “bariatric surgery, 2008–2016” and were further sub-classified based upon the type of surgery performed as previously published (Hatoum IJ JAMA Surg 2016;151; Ibrahim AM, Ghaferi AA, JAMA Surg 2017;152(7)): Roux-en-Y gastric bypass (roux), sleeve gastrectomy (sleeve), and gastric banding (band) (see Supplemental Information for included procedures in each category). Patients who had an ICD-9 or ICD-10 code for a history of a bariatric surgery and who did not have any bariatric surgical procedure code during the study period were defined as “bariatric surgery before 2008.” These patients were not sub-classified by surgical type due to an inability to determine procedure type in the absence of procedure codes. Alcohol-related cirrhosis (AC), alcohol misuse, and all comorbidities were defined by ICD-9 and ICD-10 codes, as previously published 12. Elixhauser comorbidity scores were calculated, excluding the liver, alcohol, hypertension, and diabetes categories as these were used as independent predictors of outcomes in main effects and interaction analyses.
Statistical Analysis
We used Cox proportional hazards regression models to establish risk factors for each outcome of interest (AC and alcohol misuse). All analyses were performed in weighted data. The time variable for the hazard regression was age, thus the baseline hazard function represents the age-specific hazard for the outcome. Time-varying risk factors, meaning those covariates that change during the study period (such as the development of ascites or variceal bleeding, for example), were updated when they appeared in the claims records. Entry and exit times were defined as January 1st/December 31st of the first/last year in which a subject had at least 360 days of coverage, and for which all intervening years between the entry and exit date also had ≥360 days of coverage. If a subject had multiple disconnected intervals of coverage, the longest continuous interval was used. To reduce classification errors early in a subject’s claims record, those with a cirrhosis diagnosis within the first year after study entry were implicitly dropped. Those who had cirrhosis codes or alcohol misuse codes appear before the bariatric surgery codes were censored and not included in the analysis.
To further explore the finding of increased AC in those with bariatric surgery prior to 2008 as well as increased alcohol use overall, we performed interaction analyses for bariatric surgery prior to 2008 and gender, adjusting for diabetes, hypertension, and Elixhauser score. Using interactions, we fit models in which each of these groups has its own hazard ratio relative to the baseline hazard function (for example, men have a higher baseline hazard of developing AC compared to women). Therefore, comparisons were generated between men and women with and without bariatric surgery as well as within groups (men without versus men with bariatric surgery; women without versus women with bariatric surgery). To aid in interpreting associations for interaction terms, the fitted log hazard for each combination of these interaction variables was calculated. We also performed a sensitivity analysis of our outcomes in those with hepatitis C diagnosis codes using the same statistical analysis as detailed above, as well as a sensitivity analysis of unadjusted incidence of AC and alcohol misuse per 100 person-years.
This study was deemed exempt by the University of Michigan Institutional Review Board. Patients and the public were not involved in the design or conduct of this research.
Results
Characteristics of the study population
A total weighted population of 7,015,591 was included (see Table 1). At entry into the study, mean age was 44.1 years and 61.3% were women. Mean follow-up time in the study was 3.7 years. Based on diagnosis codes, the weighted proportions were as follows: 26.4% had diabetes, 57.2% had hypertension, 0.29% had cirrhosis (0.07% AC and 0.22% non-AC), and 1.9% had documented alcohol misuse. The mean Elixhauser score was 1.4. 209,090 (3.0%) had a history of bariatric surgery before 2008 while 194,130 (2.7%) had a bariatric surgical procedure during the study period. Eighty percent of all bariatric surgeries (both before and after 2008) were performed in women. Among the bariatric procedures performed during the study period from 2008–2016, Roux-en-Y gastric bypass was the most common (see Figure 1). During the study period, 4,774 (0.03%) received a new diagnosis code of AC (see Table 1).
Table 1.
Population characteristics, weighted to reflect the MarketScan population.
| Total n = 7,015,591 | Percent/Range | |
|---|---|---|
| Female | 4,303,651 | 61.3% |
| Age (mean) | 44.1 | 18–64 |
| Elixhauser (mean) | 1.37 | -- |
| Diabetes | 1,851,001 | 26.4% |
| Hypertension | 4,011,850 | 57.2% |
| Follow-up time (mean years) | 3.7 | -- |
| Bariatric surgery from 2008–2016 | 194,130 | 2.77% |
| Roux-en-Y Gastric Bypass | 102,385 | 1.46% |
| Sleeve Gastrectomy | 64,687 | 0.92% |
| Laparoscopic Gastric Banding | 27,058 | 0.39% |
| Bariatric surgery before 2008 | 209,090 | 3.0% |
| Alcohol-related Cirrhosis | 4,774 | 0.03% |
| Non-alcoholic-related Cirrhosis | 15,192 | 0.22% |
| Alcohol misuse | 132,096 | 1.89% |
Impact of bariatric surgery on risk of AC
In the primary hazard regression, for those with surgery during the study period, there was no association between receiving a Roux-en-Y gastric bypass and increased risk of AC, but for those patients who received a sleeve gastrectomy or gastric banding, there was a decreased hazard ratio for developing AC (0.40; p<0.001 and 0.43, p=0.02, respectively). Patients who had a history of a bariatric surgery before 2008 had an increased risk of AC (HR 1.31, p=0.008) (See Table 2 and Figure 2). There were interaction effects for AC risk with bariatric surgery before 2008 and gender (see Table 2). Thus, women with a history of bariatric surgery had a >2-fold risk of AC (HR 2.1, 95% confidence interval 1.79–2.41) compared to women without bariatric surgery. An increase in risk of AC was also observed in men but the impact was attenuated (HR 1.3, 95% confidence interval 1.07–1.61) (see Figure 2). Although bariatric surgery prior to 2008 had a greater effect on AC in women, women still had a lower risk of AC compared to men (HR 0.46, 95% confidence interval 0.37–0.61).
Table 2.
Main effects and interaction effects of bariatric surgery on risk of alcohol-related cirrhosis.
| Variable | HR (Confidence interval) | P value |
|---|---|---|
| Bariatric Surgery from 2008–2016 | ||
| Roux-en-Y gastric bypass | 0.98 (0.76–1.23) | 0.78 |
| Sleeve gastrectomy | 0.40 (0.24–0.65) | <0.001 |
| Banded gastroplasty | 0.43 (0.21–0.87) | 0.02 |
| Bariatric Surgery before 2008*a | 1.31 (1.07–1.61) | <0.001 |
| Female* | 0.30 (0.28–0.32) | <0.001 |
| Elixhauser | 1.38 (1.37–1.40) | <0.001 |
| Diabetes | 0.94 (0.87–1.00) | 0.05 |
| Hypertension | 1.56 (1.44–1.68) | <0.001 |
Comparisons are to patients without any history of bariatric surgery.
indicates an interaction effect is present.
Figure 2.
Interaction effects in risk of alcohol misuse and AC for men and women: between group and within group comparisons.
Impact of bariatric surgery on alcohol misuse
In the secondary outcome analysis, undergoing a gastric bypass procedure after 2008 was associated with an increased risk of clinically relevant alcohol misuse across all procedure types. The risk was greatest for patients who had a Roux-en-Y gastric bypass (HR 1.86, p<0.001). A smaller but still significant increase was observed after sleeve gastrectomy (HR 1.35, p<0.001) but not after gastric banding (HR 1.09, p=0.07). Receipt of bariatric surgery before 2008 was associated with an increased risk of documented alcohol misuse (HR 1.52, p<0.001), with a greater impact in women (HR 1.98, 95% confidence interval 1.93–2.04) than in men (HR 1.53, 95% confidence interval 1.45–1.60) (see Figure 2).
Sensitivity analyses
We performed a sensitivity analysis evaluating the risk of AC and alcohol misuse for those with hepatitis C (see Supplemental Table 1). In this analysis, there was no association between receipt of a bariatric surgical procedure before or after 2008 and AC. We performed additional evaluation of interactions in the HCV positive population between gender and bariatric surgery before 2008. These showed no statistically significant increased risk for AC (For women: HR 1.37, 95% confidence interval 0.89–2.12; for men: HR 1.60, 95% confidence interval 0.98–2.61). There was also no increased risk of alcohol misuse amongst HCV positive patients with a bariatric surgical procedure. An interaction analysis showed that HCV positive women with a history of bariatric surgery before 2008 were slightly more likely to have an alcohol misuse problem compared to HCV positive women without bariatric surgery (HR 1.36, 95% confidence interval 1.11–1.66).
In another sensitivity analysis, the unadjusted incidence rates for a new diagnosis of AC were determined for those with and without bariatric surgery. The unadjusted rates of AC for bariatric surgery before 2008 were 0.05 per 100 person-years follow-up for women and 0.09 per 100 person-years for men. For bariatric surgeries after 2008, the unadjusted rates for women and men were 0.02 and 0.05 per 100 person-years, respectively.
Discussion
In this large study of privately insured adult US patients, undergoing bariatric surgery during the study period was associated with an increased risk of alcohol misuse, a lower risk of AC in the short-term but potentially, a longer-term increased risk of AC. These longer-term risks were more pronounced in women than in men. Those who had a bariatric surgery during the study period were at markedly increased risk of alcohol misuse, particularly if they had a Roux-en-Y gastric bypass.
Our study showed a decreased risk of AC in the cohort who had bariatric surgery (sleeve gastrectomy and laparoscopic banding) from 2008–2016. This is not surprising for a number of reasons. First, patients with obesity who meet criteria for bariatric surgery, particularly for Roux-en-Y gastric bypass, frequently have features of the metabolic syndrome, which increases their risk of having non-alcoholic fatty liver disease or non-alcoholic-steatohepatitis (NASH)16. In these patients, weight loss of 5–10% can result in marked reduction in the fat and inflammation content of the liver parenchyma, reducing the risk of cirrhosis development in the near-term17. Second, cirrhosis typically takes years to develop, thus the shorter follow-up time for those with more recent bariatric surgery may bias results against cirrhosis development. In our analysis of patients with a history of bariatric surgery prior to 2008 (but no surgical intervention during the study period), there was increased risk for AC, particularly amongst women who had bariatric surgery compared to those who did not. Though this data should be interpreted with caution as we do not know when or what type of bariatric surgery was performed, this link is made plausible by the increased risk of alcohol misuse after bariatric surgical procedures found in our data and confirmed in multiple other studies in the literature. We were unable to ascertain the timing or type of bariatric surgeries prior to 2008 to determine whether progression to AC is accelerated, but compared to obese patients who never had bariatric surgery, the risk was increased. Prior to 2008, trends in bariatric surgery changed from favoring simple restrictive surgeries (such as banded gastroplasty) in 67% of weight-loss surgeries in the late 1980s to 94% of patients undergoing more complex mixed restrictive-malabsorptive surgeries (such as Roux-en-Y gastric bypass) by the 1990s18. Given that alcohol misuse rates in Roux-en-Y gastric bypass have been reported to be two times higher compared to gastric banding procedures, this could suggest a potential mechanism for the increase seen in our data19. In sum, the weight loss associated with bariatric surgery may explain the short-term decreased in cirrhosis risk, but the longer-term increased risk of alcohol misuse may predispose to increased risk of AC.
Our findings of markedly increased risk of alcohol misuse, even in the relatively shorter follow-up period for those with surgery during the study period, suggests that alcohol misuse problems occur early and could contribute to the potential long-term increased risk of AC. Changes in alcohol metabolism following gastric bypass may play a role in this increased risk. Alcohol metabolism in the body occurs predominantly in the liver, where hepatic alcohol dehydrogenase metabolizes most of the consumed alcohol. Some alcohol metabolism occurs in the gastric mucosa, such that bypassing the stomach may result in increased hepatic delivery of alcohol. In a small cross-over study of 19 patients who underwent Roux-en-Y gastric bypass, peak blood alcohol concentrations after a standard dose of alcohol were substantially higher six months after the procedure compared to pre-operative alcohol levels6. Another small study of eight women who had Roux-en-Y gastric bypass surgery reported similar findings, showing higher postoperative peak blood alcohol concentrations, faster time to peak blood alcohol levels, and more pronounced, longer-lasting feelings of drunkenness7. Findings for sleeve gastrectomy or gastric banding have, in the past, been less consistent, but recent data has suggested that, similar to Roux-en-Y gastric bypass, sleeve gastrectomy also causes higher and faster peak blood alcohol concentrations 20–23However, alcohol metabolism changes are only a partial explanation. Changes in brain reward processing post-bariatric surgery have also been noted in animal models of alcohol consumption following Roux-en-Y gastric bypass, supporting the hypothesis that alcohol misuse may substitute for maladaptive behaviors following bariatric surgery 21.
Our findings of increased post-operative alcohol misuse are consistent with existing studies linking bariatric surgery, particularly Roux-en-Y gastric bypass, and increased postoperative alcohol misuse [19,21. Lifetime prevalence of Axis I psychiatric and substance use disorders in bariatric surgery patients may be as high as 73% and 32%, respectively24–26. A large retrospective Swedish study found a two-fold increased risk of inpatient care for alcohol dependence in those with Roux-en-y gastric bypass compared to those who with restrictive bariatric surgery27. A recent meta-analysis of alcohol use disorder (AUD) both before and after bariatric surgery found no significant increase in formal AUD diagnoses at one-year post surgery, but did find an 82% increase at three years (OR 1.82, CI 1.53–2.18). Our study involving nearly 400,000 persons with bariatric surgery and a mean follow-up time of 3.7 years, showed that alcohol misuse increased after bariatric surgery and over time, risk of AC also increased.
To capture all relevant alcohol misuse problems, we used an inclusive definition of alcohol misuse that included alcohol use disorder, as well as broader diagnoses of alcohol intoxication, alcohol withdrawal, alcohol-related pancreatitis, or alcohol-related liver disease. With the recognition that hazardous drinking is not uncommon before bariatric surgery and increases afterward, several guidelines recommend careful pre-operative psychosocial screening for substance use disorder and psychiatric illness along with post-operative recommendations that high-risk patients fully abstain from alcohol misuse 3. These guidelines were issued in 2012, while our study spanned 2008–2016 4. Thus, persons who had bariatric surgery prior to entry and those who had procedures during the study period may not have undergone screening pre-operatively or received post-operative counseling.
Our finding that bariatric surgery has a greater long-term impact on risk of alcohol misuse and AC in women has not been described to date, but, as discussed above, these results should be interpreted with caution and will require further validation. Women are more susceptible to the toxic effects of alcohol at lower doses. Women have less gastric alcohol dehydrogenase compared to men and a smaller volume of distribution of alcohol, which may explain the gender variation in alcohol’s effects 28. Women develop AC and alcoholic hepatitis with less total alcohol consumption as well as a shorter duration of alcohol consumption compared to men 29–31. These findings have led to recommendations for limits of safe alcohol consumption in women being half that of men (www.niaaa.org). Our finding of a more marked increased risk of AC in women suggests that women may be affected to a greater degree by the changes in alcohol metabolism after bariatric surgery. Apart from differences in alcohol metabolism, there are other gender differences in alcohol use. Women in the general population as well as those undergoing bariatric procedures are more affected by anxiety and depressive disorders 32 than men which may predispose them to alcohol misuse 24. Alcohol problems more commonly go undetected in women, and women are less likely to access alcohol treatment 33. In addition, an increase in alcohol misuse and AC among women is not limited to those who underwent bariatric surgery but is increasingly recognized in the general population, where AUDs have increased 80% in women, compared to 30% in men, and where AC rates in women are on the rise 12,34,35.
There are several limitations to our study. We used observational claims data with ICD-9/ICD-10 coding, which, though specific, may lack sensitivity. Second, pre-existing obesity and metabolic abnormalities may increase the risk of cirrhosis. Successful weight loss after bariatric surgery is associated with reduced risk of NASH cirrhosis; however, we do not have data on BMI or body composition to determine if those with a new AC diagnosis were less likely to maintain weight loss. We also do not have data on post-surgical weight regain, which could also result in confounding. In addition, due to the limitations of ICD coding for fibrosis without cirrhosis, we were unable to accurately ascertain which patients may have had F2 or F3 fibrosis leading up to their surgery which may have placed them at risk for long-term cirrhosis. Alcohol and metabolic abnormalities, particularly diabetes, may have synergistic effects on liver damage, increasing likelihood of cirrhosis, particularly in those with heavy alcohol use 36. Our regression models accounted for DM and hypertension, but obesity, as ascertained by ICD-9 and ICD 10 codes, may be less accurate.
In conclusion, patients who undergo bariatric surgery are at greater risk alcohol misuse problems in the early years following their bariatric procedures, but may be at increased risk of AC in the long term, with an effect more pronounced in women. Postoperative alcohol recommendations may need to be targeted in a gender-specific fashion, and patients should be educated about potential differences in alcohol metabolism that may make them more susceptible to AC and new or worsening alcohol misuse problems. Further prospective research is necessary to confirm these findings and to determine best practices for screening and interventions for alcohol misuse before and after bariatric surgery.
Supplementary Material
Table 3.
Main effects and interaction effects of bariatric surgery on risk of alcohol misuse.
| Variable | HR (Confidence interval) | P value |
|---|---|---|
| Bariatric Surgery from 2008–2016 | ||
| Roux-en-Y gastric bypass | 1.86 (1.79–1.94) | <0.001 |
| Sleeve gastrectomy | 1.35 (1.28–1.43) | <0.001 |
| Banded gastroplasty | 1.09 (0.99–1.19) | 0.06 |
| Bariatric Surgery before 2008*a | 1.53 (1.46–1.60) | <0.001 |
| Female* | 0.45 (0.44–0.45) | <0.001 |
| Elixhauser | 1.19 (1.19–1.20 | <0.001 |
| Diabetes | 0.91 (0.90–0.93) | <0.001 |
| Hypertension | 1.19 (1.17–1.21) | <0.001 |
Comparisons are to patients without any history of bariatric surgery.
indicates an interaction effect is present.
Acknowledgments
Financial Support: Drs. Mellinger and Fernandez are supported by an NIAAA K23 Career Development Award (AA 026333-01 and AA023869-06)
Abbreviations
- AC
alcohol-related cirrhosis
- ALD
alcohol-related liver disease
- AUD
alcohol use disorder
- HCV
hepatitis C
- ICD
International Classification of Diseases
- NASH
non-alcoholic steatohepatitis
Footnotes
Conflict of Interest: The authors disclosed no conflict of interest.
References
- 1.Adams TD, Davidson LE, Litwin SE, et al. Weight and Metabolic Outcomes 12 Years after Gastric Bypass. N Engl J Med 2017;377:1143–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lassailly G, Caiazzo R, Buob D, et al. Bariatric Surgery Reduces Features of Nonalcoholic Steatohepatitis in Morbidly Obese Patients. Gastroenterology 2015;149:379–88. [DOI] [PubMed] [Google Scholar]
- 3.Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient−−2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surg Obes Relat Dis 2013;9:159–191. [DOI] [PubMed] [Google Scholar]
- 4.Heinberg LJ, Ashton K, Coughlin J. Alcohol and bariatric surgery: review and suggested recommendations for assessment and management. Surg Obes Relat Dis 2012;8:357–363. [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim N, Alameddine M, Brennan J, et al. New onset alcohol use disorder following bariatric surgery. Surg Endosc 2018;307:2516–2530. [DOI] [PubMed] [Google Scholar]
- 6.Woodard GA, Downey J, Hernandez-Boussard T, et al. Impaired alcohol metabolism after gastric bypass surgery: a case-crossover trial. J. Am. Coll. Surg. 2011;212:209–214. [DOI] [PubMed] [Google Scholar]
- 7.Pepino MY, Okunade AL, Eagon JC, et al. Effect of Roux-en-Y Gastric Bypass Surgery: Converting 2 Alcoholic Drinks to 4. JAMA Surg 2015;150:1096–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kochkodan J, Telem DA, Ghaferi AA. Physiologic and psychological gender differences in bariatric surgery. Surg Endosc 2018;32:1382–1388. [DOI] [PubMed] [Google Scholar]
- 9.Szabo G. Women and alcoholic liver disease - warning of a silent danger. Nature Reviews Gastroenterology & Hepatology 2018;12:231–254. [DOI] [PubMed] [Google Scholar]
- 10.Erol A, Karpyak VM. Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug Alcohol Depend 2015;156:1–13. [DOI] [PubMed] [Google Scholar]
- 11.Otete HE, Orton E, West J, et al. Sex and age differences in the early identification and treatment of alcohol use: a population-based study of patients with alcoholic cirrhosis. Addiction 2015;110:1932–1940. [DOI] [PubMed] [Google Scholar]
- 12.Mellinger JL, Shedden K, Winder GS, et al. The High Burden of Alcoholic Cirrhosis in Privately Insured Persons in the United States. Hepatology 2018;68:872–882. [DOI] [PubMed] [Google Scholar]
- 13.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012;143:1179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandhu AT, Heidenreich PA, Bhattacharya J, et al. Cardiovascular Testing and Clinical Outcomes in Emergency Department Patients With Chest Pain. JAMA Intern Med 2017;177:1175–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wernli KJ, Brenner AT, Rutter CM, et al. Risks Associated With Anesthesia Services During Colonoscopy. Gastroenterology 2016;150:888–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belle SH, Berk PD, Chapman WH, et al. Baseline characteristics of participants in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study. Surg Obes Relat Dis 2013;9:926–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–357. [DOI] [PubMed] [Google Scholar]
- 18.Samuel I, Mason EE, Renquist KE, et al. Bariatric surgery trends: an 18-year report from the International Bariatric Surgery Registry. Am. J. Surg 2006;192:657–662. [DOI] [PubMed] [Google Scholar]
- 19.King WC, Chen J-Y, Courcoulas AP, et al. Alcohol and other substance use after bariatric surgery: prospective evidence from a U.S. multicenter cohort study. Surg Obes Relat Dis 2017;13:1392–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Changchien EM, Woodard GA, Hernandez-Boussard T, et al. Normal alcohol metabolism after gastric banding and sleeve gastrectomy: a case-cross-over trial. J. Am. Coll. Surg. 2012;215:475–479. [DOI] [PubMed] [Google Scholar]
- 21.Blackburn AN, Hajnal A, Leggio L. The gut in the brain: the effects of bariatric surgery on alcohol consumption. Addict Biol 2017;22:1540–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acevedo MB, Teran-Garcia M, Bucholz KK, et al. Alcohol sensitivity in women after undergoing bariatric surgery: a cross-sectional study. Surg Obes Relat Dis 2020;16:536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acevedo MB, Eagon JC, Bartholow BD, et al. Sleeve gastrectomy surgery: when 2 alcoholic drinks are converted to 4. Surg Obes Relat Dis 2018;14:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mühlhans B, Horbach T, de Zwaan M. Psychiatric disorders in bariatric surgery candidates: a review of the literature and results of a German prebariatric surgery sample. Gen Hosp Psychiatry 2009;31:414–421. [DOI] [PubMed] [Google Scholar]
- 25.Kalarchian MA, Marcus MD, Levine MD, et al. Psychiatric disorders among bariatric surgery candidates: relationship to obesity and functional health status. Am J Psychiatry 2007;164:328–34. [DOI] [PubMed] [Google Scholar]
- 26.Malik S, Mitchell JE, Engel S, et al. Psychopathology in bariatric surgery candidates: a review of studies using structured diagnostic interviews. Compr Psychiatry 2014;55:248–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ostlund MP, Backman O, Marsk R, et al. Increased admission for alcohol dependence after gastric bypass surgery compared with restrictive bariatric surgery. JAMA Surg 2013;148:374–377. [DOI] [PubMed] [Google Scholar]
- 28.Giard J-M, Terrault NA. Women with Cirrhosis: Prevalence, Natural History, and Management. Gastroenterol. Clin. North Am 2016;45:345–358. [DOI] [PubMed] [Google Scholar]
- 29.Becker U, Deis A, Sørensen TI, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology 1996;23:1025–1029. [DOI] [PubMed] [Google Scholar]
- 30.Taniai M, Hashimoto E, Tokushige K, et al. Roles of gender, obesity, and lifestyle-related diseases in alcoholic liver disease: Obesity does not influence the severity of alcoholic liver disease. Hepatol. Res. 2012;42:359–367. [DOI] [PubMed] [Google Scholar]
- 31.Horie Y, Yamagishi Y, Ebinuma H, et al. Obesity, type 2 diabetes, age, and female gender: significant risk factors in the development of alcoholic liver cirrhosis. Hepatol Int 2013;7:280–285. [DOI] [PubMed] [Google Scholar]
- 32.Barry LC, Allore HG, Guo Z, et al. Higher burden of depression among older women: the effect of onset, persistence, and mortality over time. Arch. Gen. Psychiatry 2008;65:172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradley KA, Boyd-Wickizer J, Powell SH, et al. Alcohol screening questionnaires in women: a critical review. JAMA 1998;280:166–171. [DOI] [PubMed] [Google Scholar]
- 34.Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry 2017;74:911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Åberg F, Helenius-Hietala J, Puukka P, et al. Interaction between alcohol consumption and metabolic syndrome in predicting severe liver disease in the general population. Hepatology 2018;67:2141–2149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.