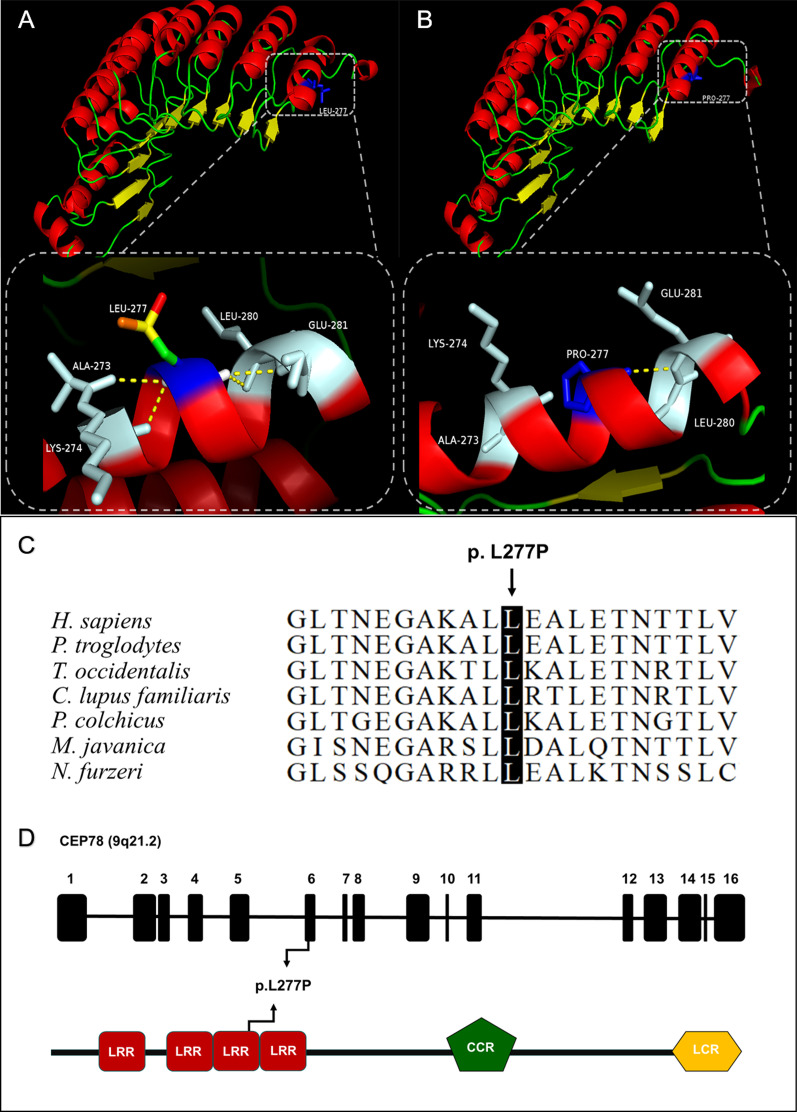
Fig. 4.
Evaluation of L277P mutation in CEP78. A Presentation of wildtype CEP78 protein 3D structure. B Mutant status of CEP78 protein. The amino acid at position 277 was mutated from leucine (LEU) to proline acid (PRO), leading to the loss of three hydrogen bonds between the wildtype LEU at residue 277 and Alanine acid (ALA) at residue 273, Lysine acid (LYS) at residue 274, and LEU at residue 280. C Alignment of CEP78 protein sequence from amino acid 267 to 287 to its orthologous protein sequences in different species indicated evolutionary conservation of leucine at position 277 in human CEP78. D Representations of relative linear locations of the L277P mutation in genome structure (top) and protein domains (bottom)