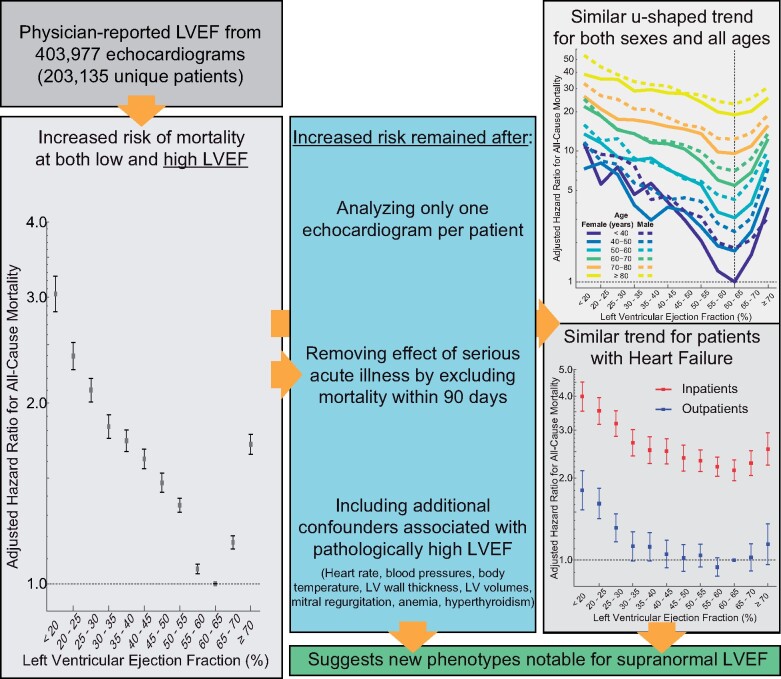
Abstract
Aims
We investigated the relationship between clinically assessed left ventricular ejection fraction (LVEF) and survival in a large, heterogeneous clinical cohort.
Methods and results
Physician-reported LVEF on 403 977 echocardiograms from 203 135 patients were linked to all-cause mortality using electronic health records (1998–2018) from US regional healthcare system. Cox proportional hazards regression was used for analyses while adjusting for many patient characteristics including age, sex, and relevant comorbidities. A dataset including 45 531 echocardiograms and 35 976 patients from New Zealand was used to provide independent validation of analyses. During follow-up of the US cohort, 46 258 (23%) patients who had undergone 108 578 (27%) echocardiograms died. Overall, adjusted hazard ratios (HR) for mortality showed a u-shaped relationship for LVEF with a nadir of risk at an LVEF of 60–65%, a HR of 1.71 [95% confidence interval (CI) 1.64–1.77] when ≥70% and a HR of 1.73 (95% CI 1.66–1.80) at LVEF of 35–40%. Similar relationships with a nadir at 60–65% were observed in the validation dataset as well as for each age group and both sexes. The results were similar after further adjustments for conditions associated with an elevated LVEF, including mitral regurgitation, increased wall thickness, and anaemia and when restricted to patients reported to have heart failure at the time of the echocardiogram.
Conclusion
Deviation of LVEF from 60% to 65% is associated with poorer survival regardless of age, sex, or other relevant comorbidities such as heart failure. These results may herald the recognition of a new phenotype characterized by supra-normal LVEF.
Keywords: Ejection fraction, Cardiac function, Survival, Mortality, Clinical practice
See page 1258 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz706)
Introduction
In patients with known or suspected heart disease, left ventricular ejection fraction (LVEF) is the most commonly used metric for quantifying ventricular function. Echocardiograms are requested for a broad population, extending from healthy patients with benign symptoms to those with severe heart failure. In 2011 alone, 7 million echocardiograms were done on one of every five of US Medicare beneficiaries.1
Despite widespread reporting of LVEF, the relationship between clinically assessed LVEF and survival has never been well defined in a large, clinical practice population. Studies have focused on patients with heart failure,2–7 have studied only a specific range (LVEF < 40%) or used a crude dichotomization (LVEF above and below 50%),2 , 7–12 and/or had only a modest sample size.4 , 5 , 8 , 9 The largest single cohort published had 8399 patients, all with an LVEF ≤40%,6 and the largest meta-analysis of trials and registries had 41 972 patients, all of whom had heart failure.13 Furthermore, patients in clinical trials may not be representative of clinical practice, and measurement of LVEF for research may differ, either because more time, effort, and expertise is applied or because the estimate is biased by a desire to enrol patients in a study where LVEF is an inclusion criterion.14 , 15 Assessment of LVEF in clinical practice, which is the measurement used to guide management, may be very different from that measured in a trial.
We hypothesized that analysis of a large clinical dataset would provide a new understanding of the relationship between clinically assessed LVEF and survival. We tested this hypothesis using data from a regional health system to capture the broad population of patients that undergo echocardiography.
Methods
Study design
All echocardiograms with physician-reported LVEF measured at rest were identified within Geisinger health records (1998–2018). Geisinger is a regional healthcare system in Pennsylvania with a catchment population of 3.5 million. Records were required to have the date of death or last living encounter and age, sex, height, and weight. Problem lists from clinics and hospitalizations were mapped to International Classification of Disease, Tenth Revision (ICD-10) codes, which were reviewed for the following diagnoses at the time of echocardiography: previous myocardial infarction, hypertension, diabetes mellitus, atrial fibrillation or flutter, congenital heart or great vessel defect, chronic kidney disease, and heart failure (Supplementary material online, Table S1). Indications for echocardiography and smoking history were obtained from structured data fields. The use of angiotensin converting-enzyme inhibitors or angiotensin-II receptor blockers, evidence-based beta-blockers, loop diuretics, mineralocorticoid receptor antagonists, and digoxin were determined within 3 months of the echocardiogram. The use of inotropic agents was determined within 1 day of the echocardiogram.
Similar echocardiographic data were gathered from the Waitemata District Health Board, the largest district health provider in New Zealand. This retrospective study complies with the Declaration of Helsinki, was approved by the Institutional Review Board, and was performed with a waiver of consent.
Left ventricular ejection fraction reporting
Many (74%) of the LVEFs were reported as a range either 4% or 5% in width (e.g. 40–44%, 55–60%). Therefore, all LVEFs were categorized into intervals 5% in width and inclusive of the lower threshold. The lowest and highest intervals were <20% and ≥70%, respectively. In addition to ranges, LVEF was reported as a single number or an inequality (e.g. <20%). A small number of reports with indeterminate inequalities were classified in the adjacent interval (e.g. <30% was categorized as 25–30%). Overall, 59% of the LVEF values were described as qualitative; 7% were derived from the bi-plane technique; 0.5% were derived from a 3D-volume technique; 0.4% were derived from a single-plane technique. The remaining 33% of LVEFs did not include a description of their derivation.
Endpoint
The endpoint was death, which was recorded in the health system when possible and otherwise checked at least monthly against national databases: the Social Security Death Index (SSDI) for Geisinger patients and Statistics New Zealand (Tatauranga Aotearoa) for the validation dataset. For Geisinger patients, the date of last living encounter within a Geisinger facility was used for censoring. For New Zealand patients, mortality was censored on 1 January 2018. Previous studies of the SSDI demonstrated that 95% of deceased patients were confirmed deceased in the SSDI.16 Under the provisions of the New Zealand Births, Deaths, Marriages, and Relationships Registration Act 1995, every death occurring in New Zealand must be registered.
Subgroup analyses
Subgroup analyses investigated features potentially associated with pathologically elevated LVEF, such as heart rate, blood pressure, body temperature, increased wall thickness, decreased left ventricular volumes, mitral regurgitation (at least moderate), anaemia (haemoglobin < 10 g/dL within 31 days of echocardiography), and hyperthyroidism [thyroid stimulating hormone (TSH) < 0.10 mIU/L within 31 days of echocardiography]. These subgroup analyses only included echocardiograms for which measurements of wall thickness, left ventricular volumes, and the severity or absence of mitral regurgitation were reported. For the subgroup of patients with heart failure, N-terminal prohormone of brain natriuretic peptide (NT-proBNP) was queried either during inpatient admission or within 6 months of echocardiography.
Statistical methods
Follow-up time was reported using the reverse Kaplan–Meier method.17 Cox proportional hazards regression was used to model time-to-mortality based on LVEF and fixed baseline confounders including age, sex, height, body mass index (BMI), smoking history, and comorbidities. Analyses from New Zealand were not adjusted for confounders because the confounder data were incomplete. For the primary analysis, all echocardiograms were included such that patients contributed all echocardiograms from different points in their lives. Those echocardiograms were treated as independent observations with the exception that clustering was performed around each patient to account for possible correlation of observations from a single patient. Robust variance estimation was performed. One sensitivity analysis included only the first echocardiogram from each patient. Another sensitivity analysis excluded echocardiograms with indeterminate LVEF as well as those performed on patients <18 years of age or with congenital heart or great vessel defects. Another sensitivity analysis allowed the interaction of exam setting (either inpatient or outpatient) with LVEF. To investigate the impact of serious acute illness, another sensitivity analysis excluded echocardiograms for which the time until either death or last encounter was <90 days. Additional analyses included interactions between LVEF and age, sex, heart failure, exam setting, and left ventricular volumetric indices. Analyses of patients with heart failure included medications as well as left ventricular volume and wall thicknesses. All analyses were performed using R (version 3.4.3) and the survival package.18 Hypothesis tests were two-tailed and used a 0.05 level of significance.
Results
Study population
A total of 596 503 echocardiograms from 271 201 patients were identified between 1998 and 2018. A total of 14 155 echocardiograms were excluded due to missing or invalid date of death (n = 608), sex (n = 200), height, weight, or BMI (n = 13 347). Physician-reported LVEF was missing for 178 371 echocardiograms; these patients had a lower prevalence of most diagnoses and had a lower risk of mortality compared to patients with a reported LVEF (Supplementary material online, Table S2). Finally, 403 977 echocardiograms from 203 135 unique patients met the inclusion criteria (Table 1, Supplementary material online, Table S3). The mean age was 64 years; 52% were men; 13% had a diagnosis of heart failure. The most commonly reported LVEF categories were 55–60% (34%) and 60–65% (26%). Chest pain (15.4%), dyspnoea/fatigue (12.7%), coronary artery disease (10.3%), aortic valve disease (9.2%), and congestive heart failure (7.9%) were the top five indications for echocardiography (Supplementary material online, Table S4). Sepsis, bacteraemia, and endocarditis together comprised 2.2%; shock and hypotension each comprised 0.3% of indications. The indication was unknown in 8.8%; a large number of uncommon indications (each <0.1%) together accounted for 7.6% of echocardiograms.
Table 1.
Baseline patient characteristics and reported left ventricular ejection fraction across the echocardiograms
| Characteristics | Primary group n (%) (N = 403 977) |
|---|---|
| Age, mean (SD), years | 63.8 (15.9) |
| Male | 208 408 (52) |
| BMI, mean (SD), kg/m2 | 30.5 (7.8) |
| Previous myocardial infarction | 38 085 (9) |
| Hypertension | 196 742 (49) |
| Diabetes mellitus | 91 464 (23) |
| Atrial fibrillation | 65 866 (16) |
| Congenital heart defect | 16 110 (4) |
| Dyslipidaemia | 176 109 (44) |
| Chronic kidney disease | 55 092 (14) |
| Heart failure | 52 192 (13) |
| Positive smoking history | 232 951 (58) |
| LVEF, mean (SD), % | 55.2 (11.1) |
| Under 20 | 5205 (1) |
| 20–25 | 7202 (2) |
| 25–30 | 8814 (2) |
| 30–35 | 9726 (2) |
| 35–40 | 10 595 (3) |
| 40–45 | 14 478 (4) |
| 45–50 | 15 889 (4) |
| 50–55 | 41 895 (10) |
| 55–60 | 138 705 (34) |
| 60–65 | 103 433 (26) |
| 65–70 | 34 508 (9) |
| Over 70 | 13 563 (3) |
BMI, body mass index; LVEF, left ventricular ejection fraction.
In the New Zealand dataset, 45 531 echocardiograms from 35 976 patients were acquired between 2008 and 2017. The mean age was 63 years (SD 17). Death occurred in 4781 patients (13.3%) who had 6375 echocardiograms (14.8%). The median follow-up time based on the reverse Kaplan–Meier method was 3.2 years [interquartile range (IQR) 1.9–4.7].
All-cause mortality
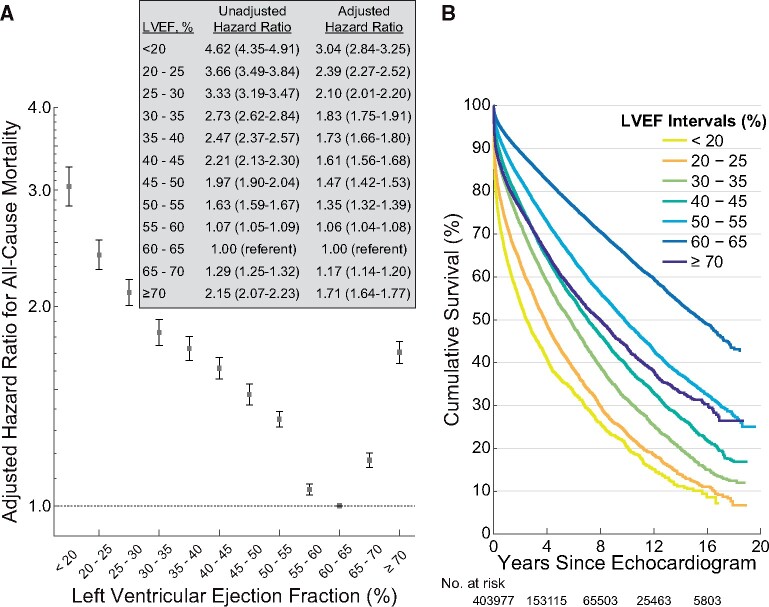
A total of 46 258 (23%) patients, who had undergone 108 578 (27%) echocardiograms, died. The median follow-up duration was 4.0 years (IQR 1.3–8.4), and 50% of echocardiograms had at least 5.2 years of follow-up until censoring or were followed until death. Adjusted LVEF hazard ratios (HRs) showed a u-shaped relationship with a nadir at 60–65%; all other LVEF intervals had significantly higher HRs (Figure 1 A). The adjusted HR for LVEF ≥70% was significantly increased [1.71 (95% confidence interval, CI 1.64–1.77)] and similar to that for LVEF 35–40% [1.73 (95% CI 1.66–1.80)]. Patients with an LVEF of 55–60% and 65–70% LVEF also had significantly higher mortality [1.06 (95% CI 1.04–1.08) and 1.17 (95% CI 1.14–1.20), respectively]. Unadjusted Kaplan–Meier estimators illustrated the stratification of survival by LVEF (Figure 1 B). Similar results, including a nadir at LVEF of 60–65%, were seen in the unadjusted HRs in the validation dataset (Supplementary material online, Figure S1).
Figure 1.
Left ventricular ejection fraction hazard ratios and Kaplan–Meier survival curves in the primary analysis (number of echocardiograms = 403 977). Left ventricular ejection fraction intervals are inclusive of the lower threshold. (A) Error bars represent the 95% confidence interval. (B) Selected left ventricular ejection fraction intervals are shown for clarity. The number at risk includes left ventricular ejection fraction intervals not shown in the figure. LVEF, left ventricular ejection fraction.
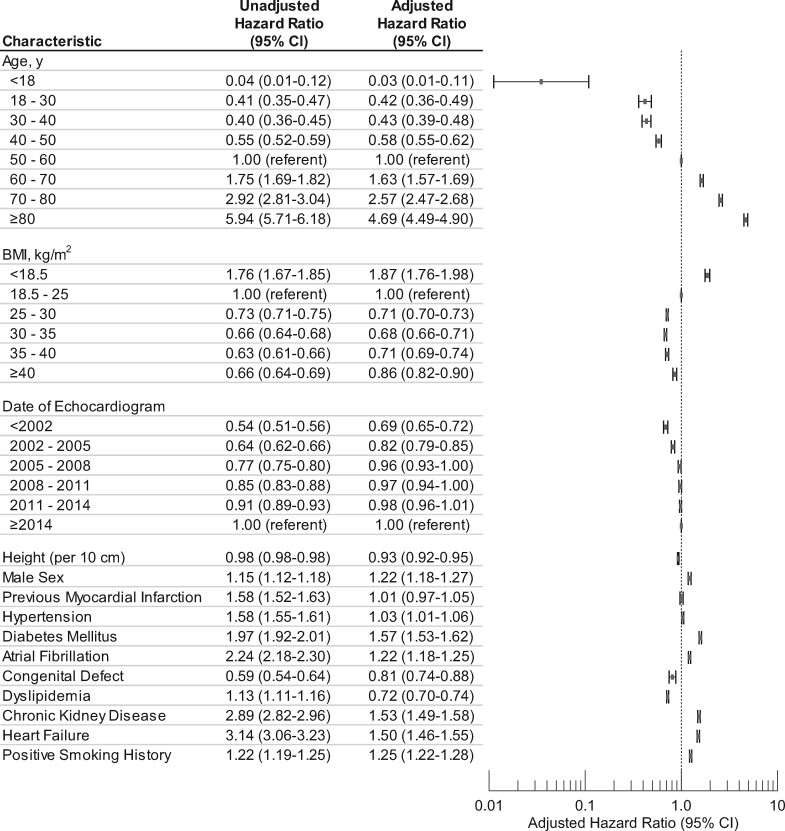
Older patients and those with diabetes, atrial fibrillation, chronic kidney disease, heart failure, and positive smoking history had higher adjusted HRs (Figure 2). Body mass index adjusted HRs had a u-shaped relationship with a nadir at 30–35 kg/m2, consistent with the ‘obesity paradox’ identified in other studies.19 , 20
Figure 2.
Confounder hazard ratios in the primary analysis (number of echocardiograms = 403 977).
Sensitivity analyses
When using only the first echocardiogram from each patient, the adjusted LVEF HRs maintained a u-shaped relationship with a nadir at 60–65% (Supplementary material online, Figure S2; baseline characteristics, Supplementary material online, Table S5). Upon excluding echocardiograms with indeterminate LVEF plus those performed on patients <18 years of age or with congenital heart or great vessel defects, the adjusted LVEF HRs were nearly unchanged (Supplementary material online, Figure S3). Using only echocardiograms with at least 90 days of follow-up, thereby excluding early deaths from serious acute illness, the adjusted HRs for the 65–70% and ≥70% LVEF intervals remained significantly elevated at 1.14 (95% CI 1.10–1.17) and 1.47 (95% CI 1.41–1.54), respectively (Supplementary material online, Figure S4). A significant u-shaped relationship was present for both inpatients and outpatients with nadirs at LVEF of 60–65% (Supplementary material online, Figure S5).
Analysis with additional entities associated with pathologically elevated left ventricular ejection fraction
In the subgroup with a more detailed echocardiographic report including wall thickness, volumes and mitral regurgitation severity, the adjusted HRs for LVEF of 65–70% and ≥70% remained significantly elevated at 1.09 (95% CI 1.05–1.12) and 1.26 (95% CI 1.21–1.33), respectively (Supplementary material online, Figure S6). Increasing septal and posterior wall thicknesses, heart rate, anaemia, mitral regurgitation, and inpatient investigation were all associated with increased adjusted HRs.
In this exploratory subgroup, the interaction (P < 0.001) between LVEF and left ventricular end-systolic volume indexed to body surface area (ESVi) demonstrated that larger volumes and reduced LVEFs had higher adjusted HRs (Supplementary material online, Figure S7). However, small ESVi (<10 mL/m2) also had higher adjusted HRs. Similar results (Supplementary material online, Figure S8) were found for the interaction between LVEF and left ventricular end-diastolic volume index (EDVi). For all but a few volume intervals with low sample sizes, the adjusted HRs for the 65–70% and ≥70% LVEF intervals remained significantly elevated compared to LVEF of 60–65%.
Analysis with interactions between age, sex, and left ventricular ejection fraction
The relationship between LVEF and survival remained u-shaped for all age groups and both sexes with nadirs at 60–65% (Figure 3). The significant interaction (P < 0.001) amongst age, sex, and LVEF reflects both the differences in the slopes of the log-scale adjusted HR curves among the age groups and the increased risk for men when LVEF was >55% but not for lower LVEFs. Furthermore, deviations from LVEF of 60–65% generally carried a greater multiplicative increase in risk for younger compared to older patients.
Figure 3.
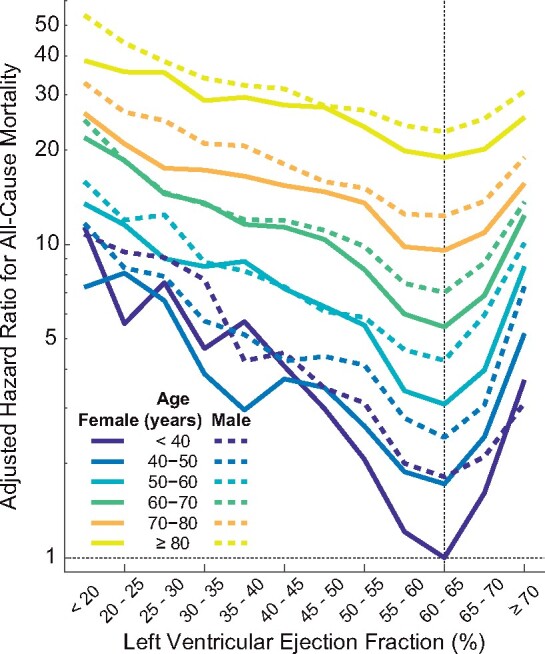
Analysis with interactions between age, sex, and left ventricular ejection fraction (number of echocardiograms = 403 977). Left ventricular ejection fraction intervals are inclusive of the lower threshold. The referent group was ‘female, under age 40 years, left ventricular ejection fraction between 60–65%’. LVEF, left ventricular ejection fraction.
Analysis in patients with heart failure
Characteristics of patients with heart failure are shown in Supplementary material online, Table S6. The relationship between LVEF and survival remained u-shaped for both inpatients and outpatients (Figure 4). Inpatients had higher adjusted HRs compared to outpatients. For inpatients, the nadir was at LVEF 60–65%. For outpatients, the nadir was at 55–60%. When a further adjustment was made for plasma concentrations of NT-proBNP in the subgroup that had such data, the strength and u-shaped relationship between LVEF and mortality appeared blunted for outpatients at both low and high LVEF and for inpatients particularly at low LVEF, although the reduced sample size and subsequently wider CIs increased uncertainty for this observation (Supplementary material online, Figure S9).
Figure 4.
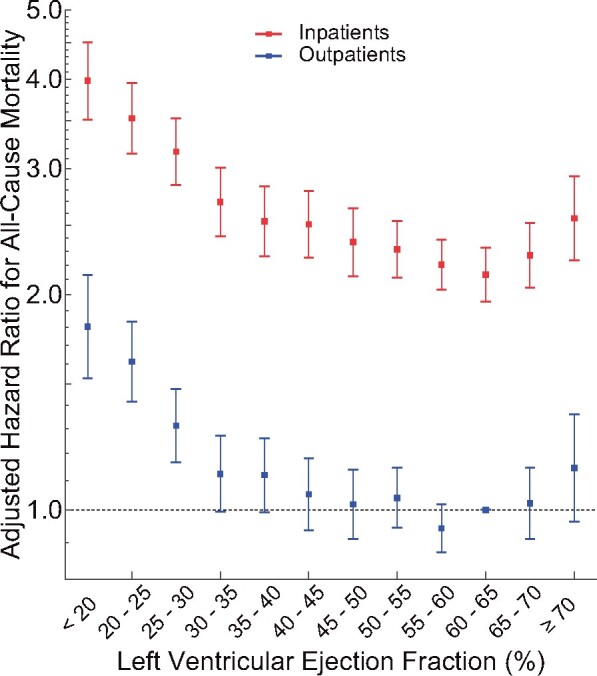
Left ventricular ejection fraction adjusted hazard ratios in patients with heart failure (number of echocardiograms = 40 616). Left ventricular ejection fraction intervals are inclusive of the lower threshold. Error bars represent the 95% confidence interval. The referent group was ‘Outpatients with left ventricular ejection fraction of 60–65%’. While 51 192 echocardiograms were performed on patients with heart failure in the primary analysis, only 40 616 echocardiograms are represented in this figure due to excluding echocardiograms missing measurements of end-diastolic volume index or wall thicknesses, for which adjustments were made in the analysis. LVEF, left ventricular ejection fraction.
Discussion
We believe this is the first analysis exploring the relationship between mortality and routinely reported echocardiographic LVEF, the result that clinicians rely on most to make decisions. Values of 60–65% were associated with the lowest mortality while both lower and higher LVEF had higher mortality as indicated by significantly higher adjusted HRs. This result was reproduced in an independent dataset from New Zealand. Deviation from this range was associated with an increase in mortality even after adjusting for numerous confounders. The 13 563 patients in our study with LVEF ≥70% had a similar mortality rate as the 10 595 patients with an LVEF of 35–40%, which could not be entirely accounted for by differences in heart rate, blood pressure, left ventricular volume, wall thickness, mitral regurgitation, anaemia, or hyperthyroidism. Among patients with heart failure, similar u-shaped relationships between mortality and LVEF were found, suggesting that it may be inappropriate to pool all patients with preserved LVEF into a single group.
An increase in mortality for LVEF ≥65% was observed across all age groups and both sexes. The trend persisted after removing echocardiograms with short follow-up to exclude deaths that may have been associated with acute illnesses such as sepsis or hypovolaemia and after adjustment for other pathologies that might increase LVEF, such as mitral regurgitation, hypertrophy, anaemia, and hyperthyroidism.21–23 It has become common practice to define ‘preserved’ as an LVEF >50% or >55%, but few studies have considered the implications of LVEF ≥65%. The nadir of the HR for incident heart failure was close to 60% in the MESA study.12 In the GRACE registry, women with acute coronary events and LVEF > 65% had higher rates of cardiac arrest or ventricular fibrillation and mortality compared to those with LVEF of 55–65%.24 U-shaped relationships between unadjusted mortality and LVEF were observed for both chronic and acute heart failure.7 , 25 The present study provides further support to the evidence that an LVEF ≥65% is associated with an increase in mortality.
For patients with heart failure, prior results have demonstrated little difference in mortality once LVEF exceeds 45%.2 In the present study, LVEF ≥ 70% predicted a higher mortality amongst both inpatients and outpatients with heart failure, as well as in the larger population without a diagnosis of heart failure, even after adjusting for many confounders, suggesting that these observations are unlikely to be spurious. Moreover, heart failure may be under-diagnosed in patients with a supra-normal LVEF as this is not currently a clinically recognized entity. The strength of the u-shaped relationships was blunted at both low and high LVEF after adjusting for plasma concentrations of NT-proBNP, particularly amongst outpatients. Regardless of LVEF, plasma NT-proBNP is usually elevated in patients with chronic heart failure regardless of LVEF, confirming cardiac dysfunction and predicting outcome. However, NT-proBNP is a much weaker predictor of outcome in the acute setting, even though plasma concentrations are often very high and patient outcomes are poor.26 Adjusting for a strong prognostic marker, such as NT-proBNP in the outpatient setting, would be expected to attenuate the relationship between LVEF and outcome. However, amongst inpatients, where it is a much weaker predictor, NT-proBNP should have little impact on the relationship between LVEF and mortality, which is what we observed. Clearly more research is needed to investigate these findings further.
The limited accuracy and reproducibility of LVEF measurements leading to potential misclassification are well-known. Undoubtedly, such measurement errors occurred in our analysis and may have contributed to the narrow range of the risk-nadir. For example, when the reported LVEF is 55–60%, rather than 60–65%, more patients will have a true LVEF of <50% due to measurement error, which may contribute to higher risk. A similar effect may operate for a reported LVEF of 65–70%. Indeed, misclassification concerns were a major reason for recent guidelines recommending the introduction of an intermediate LVEF phenotype (HFmrEF; LVEF 40–49%) as a ‘zone of uncertainty’ between heart failure with reduced LVEF (HFrEF; LVEF <40%) and heart failure with preserved LVEF (HFpEF; LVEF ≥50%).27 This zone reduces the chances of misclassification and appears to be delivering important, clinically relevant insights. Mineralocorticoid receptor antagonists (TOPCAT), angiotensin receptor blockers, (CHARM),28 and beta-blockers29 appear similarly effective for patients with HFrEF and HFmrEF but not when LVEF exceeds 50%, although there is a paucity of data on beta-blockers in HFpEF. The similar efficacy of interventions between HFrEF and HFmrEF could either be because of misclassification between these two phenotypes or simply because choosing <40% to define HFrEF was wrong; perhaps it should always have been <50%. However, our analyses suggest that risk for mortality increases even when LVEF is <60%. Given the inaccuracy of measuring LVEF for an individual patient, it may be fruitless to further refine the limit. However, this analysis suggests that a fourth left ventricular phenotype, heart failure with supra-normal LVEF (HFsnEF), might one day be recognized.
Take home figure.
Deviation of LVEF from 60–65% is associated with poorer survival regardless of age, sex or other relevant comorbidities such as heart failure. These results may herald the recognition of a new phenotype characterized by supra-normal LVEF.
Limitations
There is inherent imprecision in the clinical assessment of LVEF. However, clinically assessed LVEF, rather than LVEF measured under controlled research conditions, is the actual measurement used to guide patient care, and information on its relationship with outcomes is lacking. Selection-bias could have affected our observations, although to a much smaller extent than in a clinical trial or conventional registry, because only patients who had a clinical indication for echocardiography were included. The results of the present study are applicable to the large heterogeneous patient population referred for echocardiography. Many echocardiograms were excluded due to missing physician-reported LVEF. While these patients had fewer comorbidities, similar BMI, and a lower mortality, it remains possible that they had impaired echo views or were otherwise missing LVEF for unknown reasons.
Results from New Zealand did not include adjustment for confounders because these data were incomplete. While the unadjusted results (Supplementary material online, Figure S1) demonstrated strong similarities to the unadjusted data in the primary dataset, this limitation prevented additional sensitivity analyses similar to those performed in the primary dataset.
The study did not include advanced techniques such as 3D echocardiography, which, for example, has been recommended for evaluating valvular disease when considering surgical intervention,30 or cardiac magnetic resonance imaging (MRI), which may provide greater accuracy compared to standard 2D echocardiography. However, 2D echocardiography is the most widely used modality for assessing cardiac function. Moreover, echocardiography has been performed on a large scale for decades, and the existing datasets are orders of magnitude larger than 3D echocardiography or cardiac MRI datasets.
Potentially important confounders such as clinical status and the precise indication for echocardiography were not directly included due to either difficulty in defining an objective measure of clinical status or to the large number of specific indications which preclude adequately powered statistical analysis. The analysis with mortality censored in the first 3 months indirectly accounted for serious acute clinical status and revealed a similar u-shaped relationship and only modest decreases in the HRs for those with an LVEF of 65–70% or ≥70%, indicating that early mortality from serious acute illness may explain a small portion, but not all, of the mortality associated with a high LVEF. Some confounders that may be pertinent to heart failure, such as cardiac resynchronization therapy, were not reliably recorded and were not included.
Due to the study’s retrospective design and regression analyses, causation cannot be inferred between predictors and outcomes. Some predictors were based on diagnoses in problem lists, which may contain errors; however, the large sample size reduces the impact of uncertainty due to random errors.
Cause of death was not adjudicated in the 46 258 deaths as it would require careful chart review. The Geisinger echocardiography population was largely of European ancestry (98%), as was the New Zealand population, which limits applicability to other races and ethnicities.
Conclusion
This analysis of a large dataset found that the relationship between survival and LVEF assessed by echocardiography in routine clinical practice is u-shaped. In general, patients with an LVEF of 60–65% have the lowest risk of mortality regardless of age, sex, or other relevant confounders. Patients with LVEF ≥65% had a higher all-cause mortality. While the present analyses were retrospective with all the inherent limitations described above, our results suggest that phenotypes of HFsnEF might one day be recognized as a clinically relevant classification.
Funding
The National Institutes of Health Early Independence Award (DP5 OD012132). The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health. This project was also funded, in part, under a grant with the Pennsylvania Department of Health (#SAP 4100070267).
Conflict of interest: none declared.
Supplementary Material
References
- 1. Virnig BA, Shippee ND, O’Donnell B, Zeglin J, Parashuram S. Trends in the Use of Echocardiography, 2007 to 2011. Echocardiography Trends. Data Points #20 (prepared by the University of Minnesota DEcIDE Center, under Contract No. HHSA29020100013I). Rockville, MD: Agency for Healthcare Research and Quality; May 2014. AHRQ Publication No. 14-EHC034-EF. [Google Scholar]
- 2. Solomon SD, Anavekar N, Skali H, McMurray JJV, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, Pfeffer MA. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005;112:3738–3744. [DOI] [PubMed] [Google Scholar]
- 3. Bart BA, Shaw LK, McCants CB, Fortin DF, Lee KL, Califf RM, O'Connor CM. Clinical determinants of mortality in patients with angiographically diagnosed ischemic or nonischemic cardiomyopathy. J Am Coll Cardiol 1997;30:1002–1008. [DOI] [PubMed] [Google Scholar]
- 4. Giannuzzi P, Temporelli PL, Bosimini E, Silva P, Imparato A, Corrà U, Galli M, Giordano A. Independent and incremental prognostic value of Doppler-derived mitral deceleration time of early filling in both symptomatic and asymptomatic patients with left ventricular dysfunction. J Am Coll Cardiol 1996;28:383–390. [DOI] [PubMed] [Google Scholar]
- 5. Juillière Y, Barbier G, Feldmann L, Grentzinger A, Danchin N, Cherrier F. Additional predictive value of both left and right ventricular ejection fractions on long-term survival in idiopathic dilated cardiomyopathy. Eur Heart J 1997;18:276–280. [DOI] [PubMed] [Google Scholar]
- 6. Solomon SD, Claggett B, Desai AS, Packer M, Zile M, Swedberg K, Rouleau JL, Shi VC, Starling RC, Kozan Ö, Dukat A, Lefkowitz MP, McMurray JJV. Influence of ejection fraction on outcomes and efficacy of sacubitril/valsartan (LCZ696) in heart failure with reduced ejection fraction: the prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure. Circ Heart Fail 2016;9:e002744.. [DOI] [PubMed] [Google Scholar]
- 7. Curtis JP, Sokol SI, Wang Y, Rathore SS, Ko DT, Jadbabaie F, Portnay EL, Marshalko SJ, Radford MJ, Krumholz HM. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol 2003;42:736–742. [DOI] [PubMed] [Google Scholar]
- 8. Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, Cushman M, Polak J, Gardin JM, Gersh BJ, Aurigemma GP, Manolio TA. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med 2002;137:631–639. [DOI] [PubMed] [Google Scholar]
- 9. Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol 1999;33:1948–1955. [DOI] [PubMed] [Google Scholar]
- 10. Pernenkil R, Vinson JM, Shah AS, Beckham V, Wittenberg C, Rich MW. Course and prognosis in patients > or = 70 years of age with congestive heart failure and normal versus abnormal left ventricular ejection fraction. Am J Cardiol 1997;79:216–219. [DOI] [PubMed] [Google Scholar]
- 11. Tsao CW, Lyass A, Larson MG, Cheng S, Lam CSP, Aragam JR, Benjamin EJ, Vasan RS. Prognosis of adults with borderline left ventricular ejection fraction. JACC Heart Fail 2016;4:502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yeboah J, Rodriguez CJ, Qureshi W, Liu S, Carr JJ, Lima JA, Hundley WG, Herrington DM. Prognosis of low normal left ventricular ejection fraction in an asymptomatic population-based adult cohort: the multiethnic study of atherosclerosis. J Card Fail 2016;22:763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J 2012;33:1750–1757. [DOI] [PubMed] [Google Scholar]
- 14. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 15. Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT, Yii M, Prabhakaran D, Szwed H, Ferrazzi P, Petrie MC, O'Connor CM, Panchavinnin P, She L, Bonow RO, Rankin GR, Jones RH, Rouleau J-L. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med 2011;364:1607–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huntington JT, Butterfield M, Fisher J, Torrent D, Bloomston M. The Social Security Death Index (SSDI) most accurately reflects true survival for older oncology patients. Am J Cancer Res 2013;3:518–522. [PMC free article] [PubMed] [Google Scholar]
- 17. Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996;17:343–346. [DOI] [PubMed] [Google Scholar]
- 18. Therneau TM, Grambsch PM. A Package for Survival Analysis in S, Version 2.38. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 19. Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol 2004;43:1439–1444. [DOI] [PubMed] [Google Scholar]
- 20. Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Hear Fail 2013;1:93–102. [DOI] [PubMed] [Google Scholar]
- 21. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, Thomas JD. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol 2014;63:e57–e185. [DOI] [PubMed] [Google Scholar]
- 22. Olivotto I, Cecchi F, Poggesi C, Yacoub MH. Patterns of disease progression in hypertrophic cardiomyopathy an individualized approach to clinical staging. Circ Hear Fail 2012;5:535–546. [DOI] [PubMed] [Google Scholar]
- 23. Klein I, Danzi S. Thyroid disease and the heart. Circulation 2007;116:1725–1735. [DOI] [PubMed] [Google Scholar]
- 24. Saab FA, Steg PG, Avezum Á, López-Sendón J, Anderson FA, Huang W, Eagle KA. Can an elderly woman’s heart be too strong? Increased mortality with high versus normal ejection fraction after an acute coronary syndrome. The Global Registry of Acute Coronary Events. Am Heart J 2010;160:849–854. [DOI] [PubMed] [Google Scholar]
- 25. Toma M, Ezekowitz JA, Bakal JA, O'Connor CM, Hernandez AF, Sardar MR, Zolty R, Massie BM, Swedberg K, Armstrong PW, Starling RC. The relationship between left ventricular ejection fraction and mortality in patients with acute heart failure: insights from the ASCEND-HF trial. Eur J Heart Fail 2014;16:334–341. [DOI] [PubMed] [Google Scholar]
- 26. Cleland JGF, Teerlink JR, Davison BA, Shoaib A, Metra M, Senger S, Milo O, Cotter G, Bourge RC, Parker JD, Jondeau G, Krum H, O’Connor CM, Torre-Amione G, van Veldhuisen DJ, McMurray JJ; VERITAS Investigators. Measurement of troponin and natriuretic peptides shortly after admission in patients with heart failure-does it add useful prognostic information? An analysis of the Value of Endothelin Receptor Inhibition with Tezosentan in Acute heart failure Studies (VERITAS). Eur J Heart Fail 2017;19:739–747. [DOI] [PubMed] [Google Scholar]
- 27. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, Van Der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016;37:2129–2200.27206819 [Google Scholar]
- 28. Lund LH, Claggett B, Liu J, Lam CS, Jhund PS, Rosano GM, Swedberg K, Yusuf S, Granger CB, Pfeffer MA, McMurray JJV, Solomon SD. Heart failure with mid-range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail 2018;20:1230.. [DOI] [PubMed] [Google Scholar]
- 29. Cleland JGF, Bunting KV, Flather MD, Altman DG, Holmes J, Coats AJS, Manzano L, McMurray JJV, Ruschitzka F, van Veldhuisen DJ, von Lueder TG, Böhm M, Andersson B, Kjekshus J, Packer M, Rigby AS, Rosano G, Wedel H, Hjalmarson Å, Wikstrand J, Kotecha D. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J 2018;39:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL, Roffi M, Alfieri O, Agewall S, Ahlsson A, Barbato E, Bueno H, Collet J-P, Coman IM, Czerny M, Delgado V, Fitzsimons D, Folliguet T, Gaemperli O, Habib G, Harringer W, Haude M, Hindricks G, Katus HA, Knuuti J, Kolh P, Leclercq C, McDonagh TA, Piepoli MF, Pierard LA, Ponikowski P, Rosano GMC, Ruschitzka F, Shlyakhto E, Simpson IA, Sousa-Uva M, Stepinska J, Tarantini G, Tchétché D, Aboyans V, Windecker S, Aboyans V, Agewall S, Barbato E, Bueno H, Coca A, Collet J-P, Coman IM, Dean V, Delgado V, Fitzsimons D, Gaemperli O, Hindricks G, Iung B, Jüni P, Katus HA, Knuuti J, Lancellotti P, Leclercq C, McDonagh T, Piepoli MF, Ponikowski P, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Zamorano JL, Kzhdryan HK, Mascherbauer J, Samadov F, Shumavets V, Camp GV, Lončar D, Lovric D, Georgiou GM, Linhartova K, Ihlemann N, Abdelhamid M, Pern T, Turpeinen A, Srbinovska-Kostovska E, Cohen A, Bakhutashvili Z, Ince H, Vavuranakis M, Temesvári A, Gudnason T, Mylotte D, Kuperstein R, Indolfi C, Pya Y, Bajraktari G, Kerimkulova A, Rudzitis A, Mizariene V, Lebrun F, Demarco DC, Oukerraj L, Bouma BJ, Steigen TK, Komar M, De Moura Branco LM, Popescu BA, Uspenskiy V, Foscoli M, Jovovic L, Simkova I, Bunc M, de Prada JAV, Stagmo M, Kaufmann BA, Mahdhaoui A, Bozkurt E, Nesukay E, Brecker SJD; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.