Figure 1.
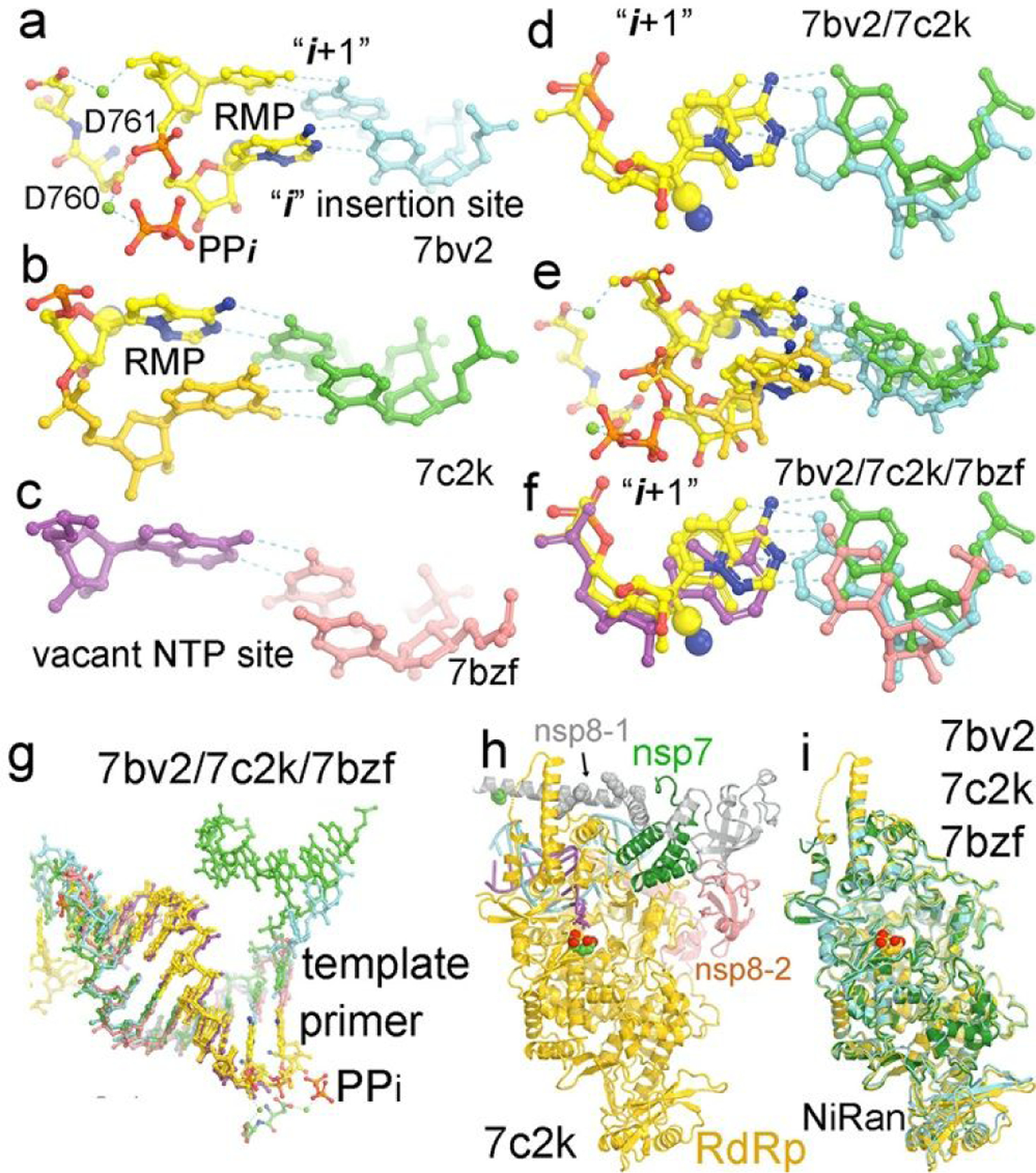
Conformations of RMP-containing primers in P/T replication complex of RdRp of SARS-CoV-2. (a) The 7bv2 structure with RMP at the “i” site and the pyrophosphate (PPi) bound. Two catalytic carboxylates D760 and D761 and two divalent metal ions are shown. (b) The 7c2k structure with RMP at the i+1 site in the pre-translocated product complex. (c) The 7bzf structure of a post-translocated product complex with a vacant NTP-binding pocket. (d) Comparison of the 7bv2 and 7c2k structures at the i+1 site. (e). Comparison of the 7bv2 and 7c2k structures at both the i and i+1 sites. (f) Comparison of the 7bv2, 7c2k and 7bzf structures at the i site. (g) Comparison of the 7bv2, 7c2k and 7bzf structures for the entire primer/template RNA duplexes. (h) The 7bv2 structure with one nsp8 in grey (nsp8–1) and the second nsp8 (nsp8–2) in salmon, nsp7 in green, and P/T in cyan, and the polymerase in gold and two catalytic carboxylates in large CPK models. (i) The superposition of the 7bv2, 7c2k, and 7bzf polymerase structures, which is a basis of comparison of corresponding P/T RNA duplexes.
