Abstract
BACKGROUND
Because of the overwhelming benefit of thrombectomy for highly selected trial patients with large vessel occlusion (LVO), some trial-ineligible patients are being treated in practice.
OBJECTIVE
To determine the safety and efficacy of thrombectomy in DAWN/DEFUSE-3-ineligible patients.
METHODS
Using a multicenter prospective observational study of consecutive patients with anterior circulation LVO who underwent late thrombectomy, we compared symptomatic intracerebral hemorrhage (sICH) and good outcome (90-d mRS 0-2) among DAWN/DEFUSE-3-ineligible patients to trial-eligible patients and to untreated DAWN/DEFUSE-3 controls.
RESULTS
Ninety-eight patients had perfusion imaging and underwent thrombectomy >6 h; 46 (47%) were trial ineligible (41% M2 occlusions, 39% mild deficits, 28% ASPECTS <6). In multivariable regression, the odds of a good outcome (aOR 0.76, 95% CI 0.49-1.19) and sICH (aOR 3.33, 95% CI 0.42-26.12) were not different among trial-ineligible vs eligible patients. Patients with mild deficits were more likely to achieve a good outcome (aOR 3.62, 95% CI 1.48-8.86) and less sICH (0% vs 10%, P = .16), whereas patients with ASPECTS <6 had poorer outcomes (aOR 0.14, 95% CI 0.05-0.44) and more sICH (aOR 24, 95% CI 5.7-103). Compared to untreated DAWN/DEFUSE-3 controls, trial-ineligible patients had more sICH (13%BEST vs 3%DAWN [P = .02] vs 4%DEFUSE [P = .05]), but were more likely to achieve a good outcome at 90 d (36%BEST vs 13%DAWN [P < .01] vs 17%DEFUSE [P = .01]).
CONCLUSION
Thrombectomy is used in practice for some patients ineligible for the DAWN/DEFUSE-3 trials with potentially favorable outcomes. Additional trials are needed to confirm the safety and efficacy of thrombectomy in broader populations, such as large core infarction and M2 occlusions.
Keywords: Computed tomography, Perfusion imaging, Acute stroke, Thrombectomy
ABBREVIATIONS
- BEST
Blood pressure for endovascular stroke treatment
- CI
confidence interval
- CT
computed tomography
- ICH
intracerebral hemorrhage
- LVO
large vessel occlusion
- LKN
last known normal
- rCBF
relative cerebral blood flow
- sICH
symptomatic intracerebral hemorrhage
The American Heart Association/American Stroke Association recommends endovascular thrombectomy for patients with acute ischemic stroke because of a large vessel occlusion (LVO) with favorable unenhanced computed tomography (CT) imaging if they present within 6 h of symptom onset.1 After 6 h, perfusion imaging is recommended to select candidates for endovascular intervention if an LVO is present.
Using RAPID automated software (iSchemaView, Redwood, California), 2 randomized clinical trials have proven a robust benefit of thrombectomy in patients with proximal LVO (ICA-terminus or M1) who present within 24 h after the time last known normal (LKN).2,3 Given the robust benefit seen in the late thrombectomy trials, some centers are treating patients who would not have been
eligible for these reasons in clinical practice. In the present subgroup analysis of the Blood Pressure for Endovascular Stroke Treatment (BEST) Study, we sought to determine if DAWN or DEFUSE-3 trial ineligibility was associated with poorer outcomes compared to patients who were trial eligible. We also compared the DAWN and DEFUSE-3 ineligible patients in our cohort to untreated controls from the DAWN and DEFUSE-3 trials.
METHODS
Patient Selection
A prospective, multicenter, observational cohort of consecutive patients over 18 yr of age with acute LVO treated with thrombectomy in routine clinical practice was used in this analysis.4 The primary purpose of the BEST study was to evaluate the impact of periprocedural blood pressure changes on clinical outcomes in patients with anterior LVO who undergo thrombectomy. To briefly summarize the BEST study methodology, consecutive patients with anterior circulation LVO (including ICA, M1, and M2) who underwent endovascular thrombectomy at 12 Comprehensive Stroke Centers in the United States (11/2017-09/2018) were prospectively enrolled and followed for 3 mo in this observational study. Patients were ineligible for inclusion if they had (1) premorbid modified Rankin Scale (mRS) score >2, (2) left ventricular assist device, (3) stroke in an inpatient or preoperative setting, or (4) terminal medical diagnosis. Patient characteristics, neuroimaging features, discharge disposition, and modified Rankin score (mRS) at 90 d were collected by local investigators using a centrally monitored, HIPAA-compliant, online platform.5 Blood pressure targets before, during, and following thrombectomy were selected at the discretion of the treating clinician at local sites and were recorded serially as part of this observational study. Ninety-day mRS scores were collected by investigators during routinely scheduled clinic visits or telephone encounters.
In this post hoc analysis, patients were included if they underwent CTP >6 h from LKN, and those with unknown LKN were excluded.
Imaging
Perfusion CT images were acquired along with CT angiography of the head and neck and were postprocessed using RAPID software to generate automated, operator-independent, motion-corrected, deconvolution-based maps of the ischemic core and hypoperfusion regions, as in recently published clinical trials.2,3,6,7 Relative cerebral blood flow (rCBF) and time-to-maximum of the tissue residue function (Tmax) were calculated automatically, based on consensus recommendations.8
Assessment of Trial Eligibility
Patients were classified as “trial eligible” or “trial ineligible” according to the inclusion and exclusion criteria of the recent DAWN3 and DEFUSE-32 clinical trials. See online Text, Supplemental Digital Content 1 for details. In the present study, patients who did not meet inclusion criteria for either trial were considered “trial ineligible,” whereas those who met criteria for one or both trials were considered “trial eligible.” Patients could have met more than 1 trial exclusion criterion.
Statistical Analysis
The primary outcome was a good functional outcome at 90 d, defined by a modified Rankin Scale (mRS) of 0 to 2. The primary safety outcome was symptomatic intracerebral hemorrhage (sICH), defined by a worsening in NIHSS of ≥4 points within 24 h of thrombectomy along with any intracerebral hemorrhage (ICH) within 72 h of thrombectomy (based on available data collected in BEST). Secondary outcomes included mTICI 2b/3 score (>50% reperfusion of the occluded target artery), 24-h NIHSS, change in NIHSS by 24 h, any ICH within 72 h of stroke onset, favorable discharge disposition (to home or rehab), shift in mRS at 90 d, and death by 90 d. Outcomes were only assessed for patients with available outcome data (missing data not imputed).
Descriptive statistics were used to compare variables of interest. Between-group comparisons were made using Chi-square or Fisher's exact test for categorical variables, or the Wilcoxon rank-sum test, where appropriate. Logistic regression was performed to estimate the association between independent variables and outcomes of interest, and these were adjusted for age, baseline NIHSS, ASPECTS, thrombolysis, and time to recanalization and was clustered by site. Ordinal logistic regression was used to estimate the association between trial eligibility and the 90-d mRS. This was adjusted for age, baseline NIHSS, ASPECTS, thrombolysis, and time to recanalization and clustered by site.
We further explored the following patient subgroups according to reasons for DAWN/DEFUSE-3 ineligibility: (1) M2 occlusions; (2) mild deficits (NIHSS <6 from 6-16 h, or <10 from 16-24 h); (3) ASPECTS <6; (4) large core (≥70 cc from 6-16 h or ≥51 cc from 16-24 h); and (5) unfavorable mismatch (defined by the DAWN clinical-imaging mismatch criteria or DEFUSE-3 target mismatch criteria, inclusive of large core volume and NIHSS criteria). Because of the anticipated small sample sizes in these subgroups, we only performed statistical testing among subgroups representing ≥20% of all trial-ineligible patients (M2 occlusions, mild deficits, ASPECTS <6, and unfavorable mismatch). In addition, we explored differences between BEST trial-ineligible patients with ICA or M1 occlusions and DAWN/DEFUSE-3 untreated controls in a sensitivity analysis. Here, the proportions of patients who experienced sICH or a good functional outcome was compared between patients who met each trial ineligibility criterion against all trial-eligible patients. We also describe outcomes for the subgroup of trial-ineligible patients with ICA or M1 occlusions and non-low NIHSS (NIHSS >6).
Multivariable logistic regression was used to estimate the odds of sICH among patients who met each ineligibility criterion against those who did not meet that criterion. Ordinal logistic regression was used to estimate the shift in 90-d mRS. Regression models were adjusted for age, baseline NIHSS (except for the subgroup of patients with mild deficits), ASPECTS (except for the subgroup with ASPECTS <6), thrombolysis, and time to recanalization and were clustered by site. No adjustments were made for multiple comparisons as all analyses were exploratory. All tests were performed at the two-sided level using STATA 15.0 (College Station, Texas). P values are provided for descriptive purposes and should be interpreted with caution.
This study was approved by the local Institutional Review Board with waiver of informed consent because of the observational nature of this study.
RESULTS
Of the 485 patients from the BEST cohort, 294 (61%) were excluded because of thrombectomy within 6 h of LKN and 2 (<1%) for missing LKN. Of the 191 who underwent thrombectomy >6 h after LKN, 93 (49%) were excluded because automated perfusion imaging was not performed. Of the remaining 98 (20%) included patients, the median age was 69 (IQR 57-81), 44 (45%) were female, and the median NIHSS was 16 (IQR 10-20).
Forty-six patients (47%) were trial-ineligible, 19 (41%) of whom had M2 occlusions, 18 (39%) had mild deficits (9 [20%] had both M2 occlusions and mild deficits), 13 (28%) had ASPECTS <6, 10 (22%) failed to meet DAWN clinical-imaging mismatch or DEFUSE-3 target mismatch criteria, 4 (9%) were treated beyond 24 h, and 3 (7%) had a core >70 cc. Compared to trial-eligible patients, trial-ineligible patients had milder baseline deficits, but also had nonsignificantly poorer baseline ASPECTS, larger core volumes, and less favorable mismatch ratios (Table 1). There were no significant differences in the overall time to recanalization or thrombolysis between the 2 groups.
TABLE 1.
Patient Characteristics
| BEST: TI n = 46 | BEST: TE n = 52 | P value (BEST-TE vs BEST-TI) | DAWN (untreated) n = 99 | DEFUSE-3 (untreated) n = 90 | P value (BEST-TI vs DAWN) | P value (BEST-TI vs DEFUSE-3) | |
|---|---|---|---|---|---|---|---|
| Age, mean (SD) or median (IQR) | 66 (58-77) | 71 (53-82) | .69 | 70.7 (13.2) | 71 (59-80) | – | – |
| No. female (%) | 19 (41%) | 25 (48%) | 50 | 48 (48%) | 46 (51%) | .43 | .27 |
| Race, no. (%) | .08 | n/a | – | ||||
| Black | 7 (15%) | 12 (23%) | NR | 5 (6%) | |||
| Caucasian | 25 (54%) | 22 (42%) | NR | 80 (89%) | |||
| Asian | 2 (4%) | 0 (0%) | NR | 3 (3%) | |||
| Other/unknown | 12 (26%) | 18 (35%) | NR | 2 (2%) | |||
| Time from last seen normal to EVT, median min (IQR) | 733 (550-990) | 725 (595-973) | .90 | NR | NR | n/a | n/a |
| Baseline NIHSS, median (IQR) | 12 (7-18) | 18 (13-21) | <.01 | 17 (14-21) | 16 (12-21) | ||
| LVO location | – | – | |||||
| ICA-T | 12 (26%) | 16 (31%) | .61 | 19 (19%) | 36 (40%) | .34 | .11 |
| M1 | 15 (33%) | 41 (79%) | <.01 | 78 (77%) | 54 (60%) | <.01 | <.01 |
| M2 | 19 (41%) | 0 (0%) | <.01 | 3 (3%) | 0 (0%) | <.01 | <.01 |
| ASPECTS, median (IQR) | 7 (5-9) | 8 (7-9) | .06 | NR | 8 (7-9) | n/a | – |
| Volume of ischemic core, median rCBF <30% mL (IQR) | 15 (0-49) | 6 (0-20) | .07 | 8.9 (3.0-18.1) | 10.1 (2.1-24.3) | ||
| Volume of perfusion deficit, median Tmax >6 s mL (IQR) | 101 (55-167) | 121 (66-157) | .57 | NR | 116.1 (73.4-158.2) | n/a | – |
| Treatment with IV tPA | 4 (9%) | 4 (8%) | 1.00 | 13 (13%) | 8 (9%) | .49 | 1.00 |
NR, TI, trial-ineligible; TE, trial-eligible; SD, standard deviation; IQR, interquartile range; EVT, endovascular thrombectomy; NIHSS, National Institutes of Health Stroke Scale; LVO, large vessel occlusion; ICA-T, internal carotid artery terminus; ASPECTS, Alberta Stroke Program Early Computed Tomography Score; IV tPA, intravenous tissue plasminogen activator.
BEST Trial-Eligible vs Trial-Ineligible Patients
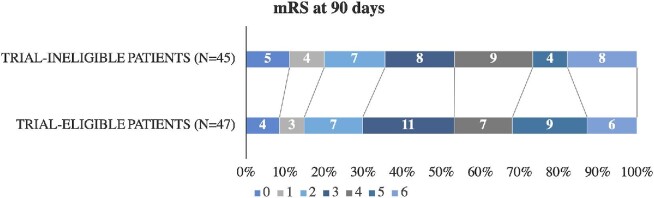
There was no difference in rates of successful recanalization (mTICI 2b/3) between trial-ineligible or trial-eligible patients (87% vs 85%, P = .74; Table 2). Ninety-day mRS scores were available for 45 trial-ineligible patients (98%) and 47 trial-eligible patients (90%). A similar proportion of trial-ineligible patients achieved the primary outcome of a good functional outcome at 90 d when compared to trial-eligible patients (36% vs 30%, P = .56). In the fully adjusted regression model incorporating age, baseline NIHSS, ASPECTS, thrombolysis, and time to recanalization, the odds of a good functional outcome remained similar among trial-ineligible patients (adjusted OR 0.76, 95% CI 0.49-1.19, P = .24). There was also no significant shift in the 90-d mRS among patients who were trial-ineligible (aOR 0.65, 95% CI 0.37-1.16, P = .15; Figure 1). In that fully adjusted model, only younger age (OR 1.05 per year, 95% CI 1.04-1.06, P < .01) and lower baseline NIHSS (OR 1.11 per NIHSS point, 95% CI 1.01-1.22, P = .02) were independently associated with better 90-d mRS.
TABLE 2.
Outcomes
| BEST: TI n = 46 | BEST: TE n = 52 | P value (BEST-TE vs BEST-TI) | DAWN (untreated) n = 99 | DEFUSE-3 (untreated) n = 90 | P value (BEST-TI vs DAWN) | P value (BEST-TI vs DEFUSE-3) | |
|---|---|---|---|---|---|---|---|
| Primary efficacy outcome | |||||||
| Good functional outcome* at 90 d | 16/45 (36%) | 14/47 (30%) | .56 | 13 (13%) | 15 (17%) | <.01 | .01 |
| Secondary efficacy outcomes | |||||||
| Successful recanalization (TICI 2b/3) | 40 (87%) | 44 (85%) | .74 | NR | NR | n/a | n/a |
| NIHSS at 24 h, median (IQR) | 11 (5-15) | 12 (5-18) | .66 | NR | NR | n/a | n/a |
| Change in NIHSS from 0 h to 24 h, median (IQR) | −1 (−4 to 3) | −5 (−11 to 0) | <.01 | NR | NR | n/a | n/a |
| Favorable discharge disposition‡ | 36 (78%) | 44 (85%) | .42 | NR | NR | n/a | n/a |
| mRS at 90 d, median (IQR) | 3 (2-5) | 3 (2-5) | .74 | NR | 4 (3-6) | n/a | – |
| Safety outcomes | |||||||
| Symptomatic ICH§ within 72 h | 6 (13%) | 2 (4%) | .14 | 3 (3%) | 4 (4%) | .02 | .05 |
| Any ICH|| | 15 (33%) | 17 (33%) | .99 | 33 (33%) | NR | 1.00 | n/a |
| Death by 90 d | 8/45 (18%) | 6/47 (13%) | .50 | 18 (18%) | 23 (26%) | 1.00 | .30 |
IQR, interquartile range; ICH, intracerebral hemorrhage; mRS, modified Rankin Scale score; NIHSS, National Institutes of Health Stroke Scale; NR, not reported; TI, trial-ineligible
*Good functional outcome indicates a mRS score of 0 to 2.
‡Favorable discharge disposition indicates discharge to home or an acute inpatient rehabilitation facility.
§In BEST, “symptomatic ICH” indicated any ICH within 72 h with a worsening in NIHSS of ≥4 points from 0 to 24 h. For DAWN, symptomatic ICH meant any extravascular blood in the cranium with a worsening in NIHSS of ≥4 points within 24 h. For DEFUSE-3, symptomatic ICH meant any ICH within 36 h with a worsening in NIHSS of ≥4 points by 36 h.
||In BEST, “any ICH” indicated any ICH within 72 h. For DAWN, this window was limited to 24 h. Because of these differences in definitions, statistical comparisons were not made.
FIGURE 1.
Ninety-day mRS according to DAWN and DEFUSE-3 eligibility. mRS denotes modified Rankin Scale. Ninety-day mRS data unavailable for 1 trial-ineligible and 5 trial-eligible patients.
There was a nonsignificantly greater proportion of trial-ineligible patients who experienced sICH when compared to trial-eligible patients (13% vs 4%, P = .14), but no difference in the proportion of patients who experienced any ICH (33% vs 33%, P = .99). After multivariable adjustment, there was no significant increase in the odds of sICH (aOR 3.33, 95% CI 0.42-26.12, P = .25), any ICH (aOR 0.74, 95% CI 0.36-1.51, P = .40), or death at 90 d (aOR 2.29, 95% CI 0.33-16.06, P = .40) among trial-ineligible patients.
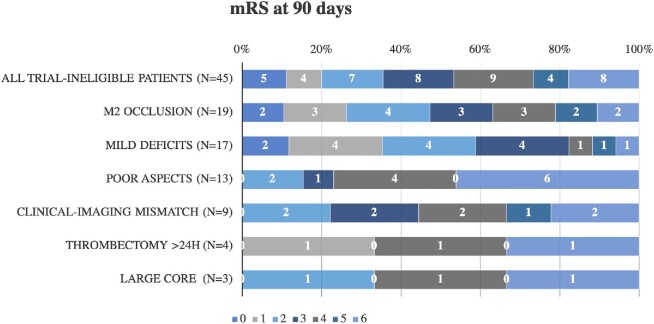
Ninety-day mRS were available for 92 patients (94% of those included) and are stratified by trial ineligibility criteria in Figure 2. Compared to patients with ICA or M1 occlusions, those with M2 occlusions had similar absolute 90-d mRS in the fully adjusted regression model (aOR for favorable 1-point shift 1.36, 95% CI 0.83-2.23, P = .23) and similar rates of sICH (aOR 1.70, 95% CI 0.35-8.36, P = .51). Compared to those without mild deficits, patients with mild deficits also had a better shift in the 90-d mRS (aOR 3.62, 95% CI 1.48-8.86, P < .01). None of the patients with mild deficits experienced sICH (0% vs 10%, P = .16). When compared to patients with good ASPECTS (6-10), those with poor ASPECTS (<6) had a poorer 90-d mRS (aOR 0.14, 95% CI 0.05-0.44, P < .01) in ordinal logistic regression, and greater odds of sICH (aOR 24.32, 95% CI 5.71-103.51, P < .01). Patients who failed to meet either DAWN clinical-imaging mismatch, or DEFUSE-3 target mismatch, had a similar 90-d mRS (aOR 0.43, 95% CI 0.11-1.66, P = .22) and a nonsignificantly greater odds of sICH (aOR 3.42, 95% CI 0.83-14.14, P = .09).
FIGURE 2.
Ninety-day mRS grouped by ineligibility criteria. Patients may have met >1 criterion for trial ineligibility. Ninety-day mRS data were available for 45 of the 46 patients, with missing data for 1 patient who otherwise would have been included among patients with mild deficits and clinical-imaging mismatch. mRS denotes modified Rankin Scale. Mild deficits indicate NIHSS <6 from 6 to 16 h after time last seen normal or <10 from 16 to 24 h. Poor ASPECTS (Alberta Stroke Program Early CT Score) indicates a score <6. Clinical-imaging mismatch indicates the patient did not meet the DAWN clinical-imaging mismatch or DEFUSE-3 target mismatch imaging profile. Large core indicates >70 cc volume using a relative cerebral blood flow <30% threshold.
BEST Trial-Ineligible Patients vs DAWN/DEFUSE-3 Controls
In comparing BEST trial-ineligible patients to untreated controls from prior trials, a larger proportion of BEST trial-ineligible patients experienced sICH (13%) than untreated DAWN (3%, P = .02) and DEFUSE-3 (4%, P = .05) controls. Despite this increase in risk, the rate of death by 90 d was similar between BEST trial-ineligible patients (18%) and untreated DAWN (18%, P = 1.00) and DEFUSE-3 (26%, P = .30) controls. A larger proportion of BEST trial-ineligible patients who underwent thrombectomy experienced a good functional outcome by 90 d (36% vs 13% [DAWN] vs 17% [DEFUSE-3], P < .05 for both).
Sensitivity Analysis
In a sensitivity analysis, comparing trial-ineligible BEST patients with ICA or M1 occlusions (n = 27) to untreated DAWN/DEFUSE-3 controls (Table, Supplemental Digital Content 2), there remained a nonsignificantly higher rate of good functional outcome by 90 d (27% vs 13% [DAWN], P = .08; 17% [DEFUSE-3], P = .25; Table, Supplemental Digital Content 3). However, the rate of sICH was significantly greater among BEST trial-ineligible patients than these untreated controls (15% vs 3% [DAWN] or 4% [DEFUSE-3], P < .05 for both comparisons). Compared to DAWN/DEFUSE-3 controls, BEST trial-ineligible patients with ICA or M1 occlusions and NIHSS >6 (n = 21) had a nonsignificantly higher rate of good functional outcome (25% vs 13% [DAWN], P = .17; vs 17% [DEFUSE-3], P = .40), whereas the rate of sICH was significantly greater among these BEST trial-ineligible patients (19% vs 3% [DAWN] or 4% [DEFUSE-3], P < .05 for both comparisons; Table 3).
TABLE 3.
Rate of Symptomatic ICH According to Trial Ineligibility Subgroup
| Rate of symptomatic ICH (%) | 90-d mRS, median (IQR) | |
|---|---|---|
| All trial-ineligible patients (n = 46) | 6 (13%) | 3 (2-5) |
| M2 occlusion (n = 19) | 2 (11%) | 3 (1-4) |
| Mild deficits (n = 18) | 0 (0%) | 2 (1-3) [n = 17] |
| Poor ASPECTS (n = 13) | 5 (38%) | 4 (4-6) |
| Clinical-imaging mismatch (n = 10) | 2 (20%) | 4 (3-5) [n = 9] |
| Thrombectomy >24 h (n = 4) | 0 (0%) | 4 (3-5) |
| Large core (n = 3) | 1 (33%) | 4 (2-6) |
IQR, interquartile range; ICH, intracerebral hemorrhage; mRS, modified Rankin Scale score.
As in Figure 2, patients may have met >1 criterion for trial ineligibility. Symptomatic ICH data were available for all patients, whereas mRS data was missing for 1 patient who met both mild deficits and clinical-imaging mismatch criteria.
Mild deficits indicate NIHSS <6 from 6 to 16 h after time last seen normal, or <10 from 16 to 24 h. Poor ASPECTS (Alberta Stroke Program Early CT Score) indicates a score <6. Clinical-imaging mismatch indicates the patient did not meet the DAWN clinical-imaging mismatch or DEFUSE-3 target mismatch imaging profile. Large core indicates >70 cc volume using a relative cerebral blood flow <30% threshold.
Compared to untreated DAWN and DEFUSE-3 controls, a greater proportion of BEST patients with mild deficits (59%) and M2 occlusions (47%) achieved a good functional outcome at 90 d (P < .05 for both comparisons). There was no difference in the incidence rate of a good outcome among patients with unfavorable ASPECTS (15% vs 13% [DAWN] and 17% [DEFUSE-3], P = ns; Figure 2). Because of the small number of patients who failed to meet the remaining eligibility criteria (clinical-imaging mismatch, treatment >24 h, and large core infarct), no formal comparisons were made between these patients and untreated DAWN/DEFUSE-3 patients.
DISCUSSION
The results from our multicenter, prospective, observational study of patients treated with thrombectomy in routine clinical practice suggest that select patients with acute anterior LVO who present within the extended window but would not have met strict trial criteria for endovascular intervention may also derive benefit from intervention. This effect appears to be driven by patients with milder symptoms and M2 occlusions; however, the effect remains significant even after adjustment for symptom severity and other outcome predictors. Furthermore, using an indirect comparison to untreated historic controls, trial-ineligible patients from BEST have better long-term functional outcomes. Although our data represent the outcomes of routine clinical practice, and are subject to selection bias and other unmeasured confounders, we have reason to be optimistic that the robust benefit of mechanical thrombectomy in acute LVO may extend beyond recommendations set forth by the AHA.1
Categorically classifying a heterogeneous population of patients as “trial-ineligible” is one major limitation of this analysis. Because “milder” patients may have driven the treatment effect observed among all trial-ineligible patients, we chose to evaluate each subgroup of DAWN/DEFUSE-3 study criteria separately. Furthermore, we performed sensitivity analyses of patients with ICA or M1 occlusions, and ICA/M1 occlusions with NIHSS >6. As expected, patients with mild presenting deficits or distal occlusions were more likely to have a better 90-d mRS without an increase in the odds of sICH.
Although patients with milder symptoms had better functional outcome with intervention, patients with more established infarctions and with clinical-imaging or target mismatch appeared to drive the poor outcome measures. Patients with ASPECTS <6 were 24 times more likely to experience sICH and 7 times more likely to have a more disabling mRS score at 90 d when compared to patients with an ASPECTS of 6 to 10. Our findings corroborate recent results from the HERMES collaborators who showed a higher risk of sICH with established infarcts on head CT.9 Unfortunately, our results are limited by a lack of adequate control arm of untreated patients with anterior LVO derived from the same observational cohort. Although we showed a limited benefit to thrombectomy in the extended window for patients with unfavorable ASPECTS in routine clinical practice (only 2/13 patients had a 90-d mRS 0-2), the HERMES collaborators observed that thrombectomy was still associated with a better 90-d functional outcome than medical management if pursued within the early window (OR 2.00, 95% CI 1.16-3.46, if ASPECTS was 3-5).9 In the extended window, other observational data suggest potential efficacy of thrombectomy in patients with LVO. One single-center study reported no significant difference in the odds of functional independence by 90 d with thrombectomy among patients with borderline ASPECTS (<7) when compared to ASPECTS of 7 to 10 (37% vs 46%, P = .852).10 Furthermore, the investigators found no increase in the risk of sICH (9% vs 9%). Our results conflict with these findings and could represent a more diverse clinical practice with a less-selected patient population. In the absence of clearer data, the risks and benefits of intervening in patients who present beyond 6 h with an unfavorable noncontrast CT should be seriously considered in future clinical trials and in clinical practice as we implement these off-label strategies.
Although our cohort represents a population that is wholly unique from DAWN/DEFUSE-3, our data also suggest that even these suboptimal candidates may also derive some benefit from thrombectomy although a comparison to patients with similar demographic features who do not receive endovascular thrombectomy is needed. Again, this benefit is largely derived by the cohort of patients with distal occlusions and milder presenting deficits. In the subgroup of patients with unfavorable baseline ASPECTS, we observed no significant benefit to endovascular intervention and a high mortality rate when compared to unmatched DAWN/DEFUSE-3 controls; however, we do not know what the mortality rate would have been without thrombectomy for these patients, and the natural history of patients with a low ASPECTS score is poor.11 It is possible that these patients may either have been selected for thrombectomy on the basis of excellent premorbid functional status or collaterals. Regardless of the indication, the functional benefit for endovascular recanalization in these patients is uncertain and may be limited.
Our data are consistent with several recent observational studies, suggesting safety and efficacy of thrombectomy in patients who would not have otherwise met DAWN/DEFUSE-3 criteria. In one single-center registry of patients with an unknown LKN, 38% of DAWN-ineligible patients (16/42) and 41% of DEFUSE-3-ineligible patients (7/17) achieved a mRS of 0 to 2 by 90 d.12 Similarly, according to observational data regarding 21 patients at 3 sites who would have met DAWN criteria but underwent thrombectomy 24 h after LKN, 5% of patients experienced sICH, and 43% achieved a 90-d mRS 0 to 2.13 In a separate study, investigators observed a 30% rate of good functional outcome (90-d mRS 0-2) among 37 included DAWN/DEFUSE-3-ineligible patients treated with off-label thrombectomy.14
Regarding the benefit of thrombectomy in patients with mild deficits, one retrospective analysis found that thrombectomy in patients with NIHSS <6 was associated with a favorable shift in 3 to 6 mo functional outcome (P < .01).15 Furthermore, after matching patients according to thrombectomy or medical management, thrombectomy remained independently associated with less long-term functional disability (P = .04). These data conflict with a propensity-matched retrospective cohort of 62 paired patients by Sarraj et al,16 in which patients with mild symptoms (NIHSS <6) and occlusion of the ICA, M1, or M2 were at similar odds of a good 90-d functional outcome (mRS 0-2) if they underwent thrombectomy or medical management (aOR 1.17; 95% CI 0.54-2.52, P = .69). Future randomized studies like ENDOLOW, SELECT, and IN EXTREMIS may eventually answer the question of whether or not thrombectomy is safe and effective in these patients.
Limitations
These data reflect the results of routine clinical practice at 12 Comprehensive Stroke Centers. This study is subject to selection bias given that only patients who underwent attempted thrombectomy were included. Participating sites in this study also did not report the number or characteristics of patients who were screened for, but did not undergo, thrombectomy, and reasons for deferral of endovascular treatment, which also contributes to this bias. Importantly, we found that more than half of patients who went to angiography would not have qualified for DAWN or DEFUSE-3 on the basis of clinical or imaging features. This indicates a growing national trend to pursue this powerful intervention in patients who do not meet strict trial criteria. In spite of this selection bias that may have favored inclusion of better candidates, we observed a lower rate of good functional outcome in our cohort than in treated patients from DAWN or DEFUSE-3. This may be due to unmeasured confounders or differences in technical expertise among proceduralists. Our definition of sICH was also not perfectly consistent with sICH definitions used in prior studies of endovascular thrombectomy17 or in the DAWN3 and DEFUSE-32 trials, in which rates of sICH were 3% to 5%. This may be explained by our overly sensitive definition of sICH, which included any hemorrhage up to 72 h after LKN as long as the patient experienced a decline in 4 or more NIHSS points. Finally, the lack of patient-level data from DAWN and DEFUSE-3 prohibited multivariable modeling for comparisons between BEST and historic controls.
CONCLUSION
In clinical practice, about half of patients undergoing thrombectomy would not have been formally eligible for the major trials, yet we found that thrombectomy in some of these highly selected patients was associated with comparable efficacy and safety to trial-eligible patients. Importantly, our results may have been driven by patients with milder symptoms and distal occlusions. These preliminary results are limited by the small sample size, heterogeneous population, and lack of an appropriate control group for comparison, and future trials targeting specific trial-ineligible candidates are required to confirm our findings.
Disclosures
This study was supported by a Society of Vascular and Interventional Neurology (SVIN) pilot grant, University of Cincinnati Gardner Neuroscience Institute pilot grant, StrokeNet NCC (U01 NS086872), and StrokeNet University of Cincinnati RCC (U10 NS086512). The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Notes
Preliminary results of this investigation were delivered via an oral platform presentation on February 6, 2019, at the 2019 American Heart Association's International Stroke Conference by the lead author (Dr Siegler) in Honolulu, Hawaii. The reference for the presentation is as follows:
Siegler JE, Messé SR, Sucharew H, Mehta T, Arora N, Starosciak AK, De Los Rios La Rosa F, Barnhill NR, Mistry A, Patel K, Assad S, Tarboosh A, Dakay K, Wagner J, Bennett A, Jagadeesan B, Streib C, Weber SA, Chitale R, Volpi JJ, Mayer S, Yaghi S, Jayaraman M, Khatri P, Mistry EA. Thrombectomy Is Safe For Dawn- And Defuse-3- Ineligible Patients Who Present Within the Extended Window: A Subgroup Analysis From The BEST Prospective Cohort Study. Oral presentation at the International Stroke Conference, Honolulu, HI. February 6th, 2019.
Supplementary Digital Content 1. Text. Methods.
Supplemental Digital Content 2. Table. Demographic measures from the sensitivity analysis, including only trial-ineligible patients with ICA or M1 occlusions. NR denotes “not reported,” TI trial-ineligible, TE trial-eligible, SD standard deviation, IQR interquartile range, EVT endovascular thrombectomy, NIHSS National Institutes of Health Stroke Scale, LVO large vessel occlusion, ICA-T internal carotid artery terminus, ASPECTS Alberta Stroke Program Early Computed Tomography Score, and IV tPA intravenous tissue plasminogen activator.
Supplementary Digital Content 3. Table. Outcome measures from the sensitivity analysis, including only trial-ineligible patients with ICA or M1 occlusions. NR denotes “not reported,” TI trial-ineligible, NIHSS National Institutes of Health Stroke Scale, IQR interquartile range, mRS modified Rankin Scale, and ICH intracerebral hemorrhage. *Good functional outcome at 90 days indicates a mRS score of 0 to 2. ‡Favorable discharge disposition indicates discharge to home or an acute inpatient rehabilitation facility. §In BEST, “symptomatic ICH” indicates the presence of any intracerebral hemorrhage within 72 h, with a worsening in NIHSS of 4 or more points from 0 to 24 h. For DAWN, this term was defined as any extravascular blood in the cranium that was associated with a worsening in NIHSS of 4 or more points within 24 h. For DEFUSE 3, this term was defined as any intracerebral hemorrhage within 36 h that was associated with a worsening in NIHSS of 4 or more points by 36 h. Despite minor differences, statistical comparisons were made between the studies. ||In BEST, “any ICH” indicates the presence of any intracerebral hemorrhage within 72 h. For DAWN, these data were only available for cases of ICH within 24 h. Because of these differences in definitions between studies, statistical comparisons were not made.
COMMENTS
This post-hoc analysis of the BEST patient cohort illustrates the potential benefit of thrombectomy in patients presenting over 6 hours from last known well with M2 occlusions, mild deficits or ASPECTS <6. These patients would be ineligible for enrollment in DAWN/DEFUSE-3. Given the remarkable treatment effect illustrated in both DAWN and DEFUSE-3 (numbers needed to treat for functional independence of 2.8 and 3.6, respectively), it is highly likely that a broader cohort of patients can benefit from thrombectomy over 6 hours from last known well.
In this analysis, patients with mild deficits (NIHSS <6 from 6-16 hrs or <10 from 16-24 hours) had a better shift in 90-day mRS and none experienced symptomatic ICH, promising results as we look to results from the upcoming “low NIHSS” trials: ENDOLOW and INEXTREMIS-MOSTE.
In our own institutional review of 2667 patients presenting with acute ischemic stroke over the 2.5 year DAWN trial enrollment period, 30% presented during the 6-24 hour time window. 22% met DAWN criteria and 23% DEFUSE-3 criteria; however, the largest subgroup of patients harbored cores too large for inclusion (greater than 70 ccs, 26%). Fortunately, as suggested by this paper and a recent post-hoc analysis of the HERMES data, a clinical benefit of thrombectomy may still be realized in patients with large cores. This is soon to be evaluated formally in the upcoming “large core” trials: IN EXTREMIS-LASTE, TESLA and TENSION.
Bradley A. Gross
Pittsburgh, Pennsylvania
The authors use a prospective, multicenter dataset from the BEST trial to examine the rate of symptomatic intracranial hemorrhage (SICH) and good 90-day outcome in patients stratified by potential eligibility in the DAWN or DEFUSE-3 trials. In addition, they compare these patients to untreated controls.
The important findings of this paper are that there are similar results in trial eligible and ineligible patients in terms of good outcome and SICH. They did find that compared to untreated controls, the trial ineligible patients had better 90-day outcomes but also increased SICH.
The major limitation of the paper is that it is a quite heterogeneous group with patients being excluded from the trials for a variety of reasons. As expected, the low NIHSS and distal occlusions did well, and the numbers in each group are quite small which limits statistical analysis. In addition, 93 patients presenting after 6 hours were excluded for lack of CTP info, which constitutes nearly half the potential patients.
Despite these limitations, this paper adds to the body of literature on thrombectomy and adds some necessary, prospectively acquired real world experience.
Jonathan Andrew Grossberg
Atlanta, Georgia
REFERENCES
- 1. Powers WJ, Rabinstein AA, Ackerson T et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2018;49(3):e46-e110. [DOI] [PubMed] [Google Scholar]
- 2. Albers GW, Marks MP, Kemp S et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nogueira RG, Jadhav AP, Haussen DC et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. [DOI] [PubMed] [Google Scholar]
- 4. Mistry EA, Sucharew H, Mistry AM et al. Blood pressure after endovascular stroke therapy (best): final results of a prospective multicenter cohort validation study. Stroke. 2019; Epub ahead of print. doi: 10.1161/STROKEAHA.119.026889. [Google Scholar]
- 5. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (redcap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goyal M, Demchuk AM, Menon BK et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019-1030. [DOI] [PubMed] [Google Scholar]
- 7. Saver JL, Goyal M, Bonafe A et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295. [DOI] [PubMed] [Google Scholar]
- 8. Wintermark M, Albers GW, Broderick JP et al. Acute stroke imaging research roadmap ii. Stroke. 2013;44(9):2628-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roman LS, Menon BK, Blasco J et al. Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol. 2018;17(10):895-904. [DOI] [PubMed] [Google Scholar]
- 10. Logan C, Maingard J, Phan K et al. Borderline Alberta stroke programme early CT score patients with acute ischemic stroke due to large vessel occlusion may find benefit with endovascular thrombectomy. World Neurosurg. 2018;110:e653-e658. [DOI] [PubMed] [Google Scholar]
- 11. Hill MD, Buchan AM. Canadian Alteplase for stroke effectiveness study (CASES) investigators. Thrombolysis for acute ischemic stroke: Results of the Canadian Alteplase for stroke effectiveness study. CMAJ. 2005;172(10):1307-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ducroux C, Khoury N, Lecler A et al. Application of the dawn clinical imaging mismatch and defuse 3 selection criteria: benefit seems similar but restrictive volume cut-offs might omit potential responders. Eur J Neurol. 2018;25(8):1093-1099. [DOI] [PubMed] [Google Scholar]
- 13. Desai SM, Haussen DC, Aghaebrahim A et al. Thrombectomy 24 hours after stroke: beyond dawn. J Neurointerv Surg. 2018;10(11):1039-1042. [DOI] [PubMed] [Google Scholar]
- 14. Desai SM, Rocha M, Molyneaux BJ et al. Thrombectomy 6-24 hours after stroke in trial ineligible patients. J Neurointerv Surg. 2018;10(11):1033-1037. [DOI] [PubMed] [Google Scholar]
- 15. Haussen DC, Lima FO, Bouslama M et al. Thrombectomy versus medical management for large vessel occlusion strokes with minimal symptoms: an analysis from STOPstroke and GESTOR cohorts. J Neurointerv Surg. 2018;10(4):325-329. [DOI] [PubMed] [Google Scholar]
- 16. Sarraj A, Hassan A, Savitz SI et al. Endovascular thrombectomy for mild strokes: how low should we go? Stroke. 2018;49(10):2398-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goyal M, Menon BK, van Zwam WH et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.