Figure 1. Conserved polarity regulators: molecular overview.
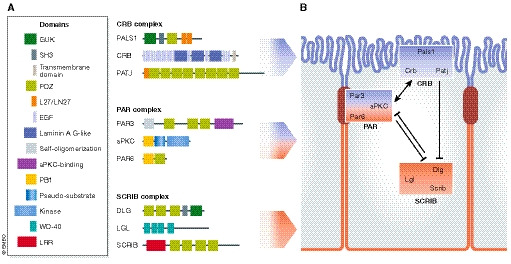
(A) Apical–basal polarity complexes in mammals. There are three main conserved polarity complexes in mammals, the apical Crumbs, the apico‐junctional Par and the basolateral Scribble complex. Together, they mediate the establishment of apico‐basal polarity. The Crumbs complex is composed of Pals1 and Pals1‐associated tight junction (PATJ) homologue proteins. The Par complex consists of partitioning‐defective 3 (Par3), partitioning‐defective 6 (Par6) and atypical kinase C (aPKC). The Scribble complex includes lethal giant larvae (Lgl), discs large (Dlg) and Scribble (Scrib) proteins. These polarity complexes consist of several scaffold proteins containing protein–protein interaction domains (e.g. PDZ) mediating the binding of different polarity proteins across the apical and basolateral domains. The kinases aPKC and Par1 (not shown) play an important role by promoting the mutual exclusion of proteins from the apical or basolateral domains (see Box 1 for details). (B) Establishment of epithelial apico‐basal polarity. The three polarity complexes, Crumbs, Par and Scribble complexes, distribute across the apical and the basolateral domain. During formation of epithelial intercellular junctions, Par3A localizes to the future tight junctions, which is required for the apical targeting of aPKC and Par6. Crumbs3 recruits Pals1, which in turns recruits Par6. When aPKC phosphorylates Par3, Par3 dissociates from aPKC/Par6. Upon Cdc42‐mediated Par6 activation, aPKC can phosphorylate Lgl1/2 and Par1 (not shown), excluding them from the apical domain. Conversely, the Ser/Thr kinase Par1b at the basolateral site phosphorylates Par3, preventing its diffusion to the basal domain.
