Figure 3. Analogies in symmetry breaking and self‐organization between early embryogenesis and epithelial tissues.
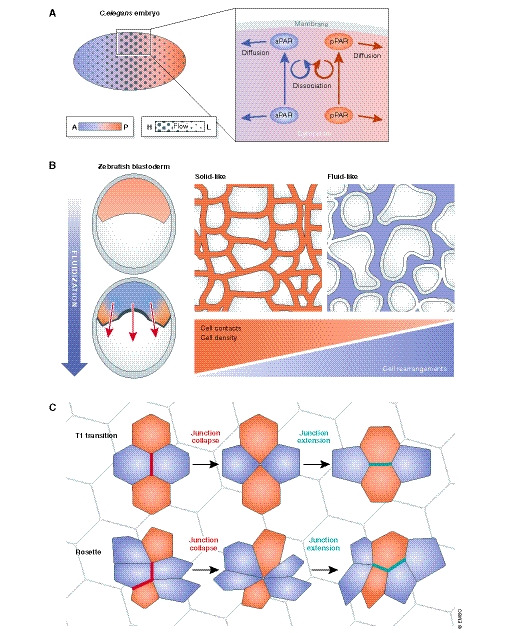
(A) In the Caenorhabditis elegans one cell embryo, establishment of anterior and posterior polarity domains is achieved by antagonistic interactions between the polarity components of both domains and contributions by anterior‐directed cortical actomyosin flow. The shear stress generated by the actomyosin flow acts on the cytoplasm and leads to the passive transport of cytoplasmic components (Illukkumbura et al, 2020). PAR network interactions maintain a polarized state, following the segregation of the aPAR to the anterior site, while the pPAR remains posterior. Polarization of the embryo is therefore the result of continuous diffusion of PAR molecules between areas where they can associate with each other and areas promoting their dissociation. This phenomenon can be described by a reaction–diffusion model, in which the PAR components transit between the cytoplasm and a membrane‐associated state where they laterally diffuse until being detached by an antagonist (Goehring et al, 2011; Rodriguez et al, 2017; Gross et al, 2019). This type of intracellular self‐organization forms the basis of various polarization events in small systems (Ganguly et al, 2012; Niwayama et al, 2016; Stückemann et al, 2017). In epithelial tissues, self‐organization manifests at a supracellular level and can drive cell sorting, tissue organization and pattern formation (Kondo & Miura, 2010; Shyer et al, 2015, 2017). (B) Tissue fluidity as an organizational principle in morphogenesis. Tissue morphogenesis results from the combination of forces driving tissue movements (e.g. pressure, collective cell movements) and the material properties of the tissue (viscoelasticity). Symmetry breaking events will initiate body axis formation (e.g. anterior–posterior, dorsal–ventral) and progressive polarization. Tissues with different viscoelastic properties will respond differentially to external forces. Despite apparent high viscosity of tissues, if a rheological threshold is reached (“yield stress”), they will undergo a “solid” to “fluid” transition and will behave like fluids. Tissue fluidity depends on cell rearrangements such as cell intercalations and turn‐over of junctional complexes. Anterior–posterior gradients of the yield stress have been implicated in body axis elongation (Mongera et al, 2018) and in gastrulation (Petridou et al, 2019) of the zebrafish. Fluidization in tissues due to mitosis or apoptotic events is thought to affect tissue viscoelasticity (Ranft et al, 2010) and tissue compression (Krajnc et al, 2018). In mammalian epithelia, state transitions have been implicated in the pathophysiology of asthma (Park et al, 2015) and fate decisions (Miroshnikova et al, 2018). (C) Intercalation is the process in which cells actively exchange their neighbours. There are two main types of planar intercalations that occur during morphogenesis: T1 transitions and rosettes. Both types require that actomyosin exerts force (i.e. cortical tension) on adherens junctions, thereby defining the junctional length (i.e. elongation or shrinking of the cell contact). Cells constantly fluctuate their cell–cell adhesions as a result of actomyosin dynamics, in order to delay or speed up cell rearrangements and consequently the neighbour exchange rate (David et al, 2014). Neighbour exchange is an essential behaviour of tissues and contributes to many processes such as tissue bending (Mason et al, 2013; Shook et al, 2018), elongation (Lienkamp et al, 2012) and wound healing, but can also modulate tissue fluidity (Rauzi et al, 2015; Curran et al, 2017; Tetley et al, 2019). There are several constraints that dictate an optimal cell arrangement. Cells must maintain their function (e.g. barrier performance), sustain their growth‐to‐differentiation balance (i.e. self‐renewal) and be able to dynamically interact with their neighbours (Tetley & Mao, 2018).
