Abstract
Background
Immune checkpoint inhibitors (ICIs) have revolutionized nonsmall cell lung cancer (NSCLC) treatment and significantly increased overall survival of patients. However, the incidence of concurrent infections and their management is still debated.
Methods
From August 2015 to October 2019, all consecutive patients with NSCLC who received nivolumab or pembrolizumab as first- or second-line therapy were retrospectively evaluated. At the time of analysis all patients had died. Clinical characteristics of patients, type of infections, and predictors of mortality were analyzed.
Results
A total of 118 patients were identified: 74 in the nivolumab group and 44 in the pembrolizumab group. At least 1 infection was recorded in 22% of the nivolumab-group versus 27% of the pembrolizumab-group (P = .178). In both groups, the main infection was pneumonia, followed by skin and soft tissue infections, urinary tract infections, and gastroenteritis. Crude mortality for first infection was 10.7%, followed by 25% and 40% for the second and third recurrence, respectively (p for trend = .146). No opportunistic infections were recorded. It is notable that, by Cox-regression model, the independent predictor of mortality was a higher Eastern Cooperative Oncology Group performance status at baseline (P < .001), whereas the multidisciplinary diagnosis and treatment of concurrent infections was associated with a reduced probability of mortality (adjusted hazard ratio = 0.50; 95% confidence interval = 0.30–0.83; P < .001).
Conclusions
In patients with NSCLC treated with ICIs, multidisciplinary management of concurrent infections may reduce the risk of mortality. Further studies to investigate risk factors for infections, as well as appropriate management strategies and preventive measures in this setting, are warranted.
Keywords: advanced lung cancer, bacterial infections, immunocompromised hosts, infectious diseases consultation, pneumonia
Immune checkpoint inhibitors have revolutionized non–small cell lung cancer treatment. In these patients, multidisciplinary management of concurrent infections with Infectious Diseases specialists may reduce the risk of mortality. Further studies to investigate risk factors for infections are warranted.
Immune checkpoint inhibitors (ICIs) have revolutionized nonsmall cell lung cancer (NSCLC) therapy, and this led to outstanding results. Clinical management of patients affected by advanced NSCLC and treated with ICIs often requires a complex and multidisciplinary approach. Many adverse events that can occur during the course of therapy should be diagnosed in a timely manner and therefore differentiated from infectious diseases that are appropriately treated [1].
In general, immune-related adverse events (IrAEs) are considered the main issues during treatment because they can potentially affect every organ or system and mimic different medical conditions [1]. In addition, the cornerstone treatment strategy of IrAEs is high-dose corticosteroid treatment, with or without other immune suppressants (such as anti-tumor necrosis factor [TNF]-α monoclonal antibody-TNFα) [2]; hence, a precise differential diagnosis should be posed with infectious diseases before starting the treatment. As a result, a deep understanding of possible infectious risk carried by different drugs, as well as the knowledge of host-related risk factors for infections (type and stage of underlying cancer, other comorbidities, previous infections, etc), should be considered for each patient before starting the ICI, as is currently done for new drugs in the field of autoimmune and idiopathic diseases [3].
Nevertheless, ICI-related infectious risk is still not well known, and studies exploring this association are lacking. Emerging concern regarding these complications have been reported since 2017 [4]; however, for the first time, Malek et al [5] explored the risk factors for infections in patients with NSCLC treated with ICIs plus chemotherapy, comparing them with a control group of patients who received treatment with cytotoxic chemotherapy alone. Malek et al [5] concluded that ICIs did not carry a specific risk for opportunistic infections; however, in both groups, 15% and 22% of patients developed at least 1 infection, respectively, whereas only 9% developed an IrAE, which suggests that infectious complications are still important adverse events in cancer patients, even if these are caused by “common” pathogens.
In addition, other studies showed a possible specific risk of tubercular reactivation [6] and other opportunistic infections, especially Pneumocystis jirovecii pneumonia (PjP), particularly in cases of corticosteroid treatment for IrAEs (with or without other immune suppressants) [7, 8], which suggests the need to raise the level of attention regarding these unexpected complications.
To the best of our knowledge, recommendations about appropriate strategies of diagnosis and treatment of concurrent infections in patients affected by NSCLC treated with ICIs are still lacking. In this study, we evaluated the incidence and type of concurrent infections in a cohort of patients affected by NSCLS and receiving ICIs; in addition, we explored predictors of mortality, including the impact of multidisciplinary management of concurrent infections in view of future multidisciplinary programs dedicated to the management of these complications.
PATIENTS AND METHODS
Study Design
From August 2015 to October 2019, all consecutive patients observed at the Thoracic Oncology Unit, IRCCS Istituto Tumori “Giovanni Paolo II” of Bari, with confirmed advanced NSCLC, who started ICIs with nivolumab (approved as second-line treatment) or pembrolizumab (approved as first- or second-line therapy), were retrospectively evaluated.
The study endpoints were as follows: (1) to describe incidence and type of infections since ICIs treatment initiation in our study population; (2) to evaluate predictors of mortality in this cohort of patients; and (3) to evaluate the impact of multidisciplinary management of infectious diseases on mortality.
Therefore, all available medical records were revised between October 2019 and June 2020 for all patients who had progressed to death. A total of 118 were dead at the time of research and were included in this study. The collected data included patients’ characteristics, comorbidity, type of lung cancer, ICI treatment duration, prior treatment, any IrAEs, and use of immunosuppressive drugs.
The Eastern Cooperative Oncology Group (ECOG) performance status (PS) at baseline was recorded for each patient [9]. According to the manufacturers’ sheets, the efficacy and safety of pembrolizumab (available at https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf) and nivolumab (available at https://packageinserts.bms.com/pi/pi_opdivo.pdf) in patients with ECOG PS >2 was not explored in clinical trials, and their use in this condition is not approved and was not performed by our Center.
In addition, all episodes of infectious diseases, instrumental diagnostic procedures (including any ultrasonography, chest x-ray, computed tomography scan, magnetic resonance, or positron emission tomography performed), microbiological investigations (blood, urine, sputum, and bronchoalveolar cultures), infectious site, microbiologic isolates, treatment performed, and outcome were recorded. Because the Cancer Institute does not have a dedicated Infectious Diseases Unit, all consultations for cancer patients with suspected or ascertained infection were performed by the Infectious Diseases (ID) specialists of the Clinic of Infectious Diseases, University Hospital of Bari. Therefore, the management of infectious events and the follow-up strategies were discussed collegially.
Definitions
Any episode of fever, colitis, diffuse skin rash, severe myalgias, or pneumonitis as well as reported signs or symptoms requiring the access to care and at least 1 diagnostic procedure (laboratory or microbiological investigations or radiologic imaging), if not directly related to cancer, was recorded as an adverse event.
Blood, sputum, and urine culture as well as all other microbiologic diagnostics (serology, urinary antigen testing) were performed according to physician’s judgement. Likewise, when a diagnosis of infection was suspected, patients underwent an ID specialist evaluation.
Microbiology isolates were evaluated to determine the clinical significance; pathogens identified but not warranting targeted therapy were defined as commensal and nonsignificant infective pathogens and were not reported in this study. Immune-related adverse events were diagnosed and treated according to current recommendations [10]. The diagnosis of infections was based on the presence of symptoms and signs compatible with a clinical syndrome, and/or microbiologic documentation, and/or imaging findings, or based on clinical improvement due to antibiotics alone, without the need for corticosteroids in the absence of confirmed microbiologic diagnosis [11].
Pneumonia was defined according to current guidelines [12], and the community or healthcare origin of infection was recorded. Patients were considered affected by pneumonia if they showed new onset of respiratory signs and/or symptoms (cough, sputum production, worsening dyspnea, increased oxygen requirement) associated with new infiltrates on chest x-ray or computed tomography, with or without positive respiratory cultures (sputum, bronchial wash, or bronchoalveolar lavage). Patients who presented with a clinical picture of pneumonia were considered to have bacterial pneumonia if the pneumonia resolved with antibiotics without corticosteroids [5]. Mortality for infection was evaluated in terms of both 28-day mortality and in-hospital mortality.
Data Analysis
All data were anonymized and collected on an electronical database. Descriptive statistics were produced for demographic, clinical, and laboratory characteristics of patients. Mean and standard deviation were obtained for normally distributed variables, median and interquartile range (IQR) were obtained for nonnormally distributed variables, and number and percentages were obtained for categorical variables.
The distribution between groups (patients treated with nivolumab versus patients treated with pembrolizumab) of outcomes and clinical and laboratory findings was analyzed by univariable parametric or nonparametric tests, Kruskal-Wallis test or Mann-Whitney U test (where appropriate) for continuous variables and with Pearson’s χ 2 test (Fisher’s exact test where appropriate) for categorical variables, according to data distribution.
To assess predictors of mortality of patients, a univariate Cox regression model was produced; a stepwise multivariable Cox regression was then applied to control for potential confounders and was adjusted for variables associated (P < .1) with endpoint at univariable analysis; collinear variables were excluded, with no further selection.
Finally, Kaplan-Meier curves estimates were also performed for variables of interest. In all cases, P < .05 was considered statistically significant. Statistical analysis was performed using STATA “Special Edition”, version 16.1 (STATA Corp., College Station, TX).
Ethical Approval and Participation
The research did not require a formal approval from the ethics committee according to the Italian law because it was performed as an observational retrospective study in the context of normal clinical routines (art.1, leg. decree 211/2003). However, the study was conducted in accordance with the Declaration of Helsinki and national and institutional standards.
Patient Consent Statement
All patients provided informed consent for the use of their data for research purposes. Data were previously anonymized, according to the requirements set by Italian Data protection Code (leg. Decree 196/2003).
RESULTS
General Characteristics of the Study Population
A total of 118 subjects, with a median age of 68 (IQR, 66–70) years and 21% female, were enrolled; 74 (63%) patients were treated with nivolumab and 44 (37%) patients were treated with pembrolizumab. In 64 (55%) patients, an ICI was prescribed for lung adenocarcinoma and in 53 (45%) an ICI was prescribed for squamous cell carcinoma. Of note, all patients undergoing nivolumab were previously treated with conventional platinum-based chemotherapy, whereas pembrolizumab was used as a first-line treatment in 30 patients (68% of cases).
Median follow up, corresponding to median overall survival from first dose of ICI until death, was 6 (3–11) months. Detailed characteristics of patients are shown in Table 1.
Table 1.
General Characteristics of the Study Population
| Characteristics | Total (Nr. 118) | Nivolumab (Nr. 74) | Pembrolizumab (Nr. 44) | P Value |
|---|---|---|---|---|
| Median age (IQR), yr | 68 (66–70) | 69 (66–71) | 67 (63–71) | .549 |
| Female sex, n (%) | 25 (21) | 14 (19) | 11 (25) | .434 |
| Smoking, n (%) | ||||
| Active smoker | 41 (36) | 30 (42) | 11 (26) | .252 |
| Exsmoker | 66 (58) | 38 (53) | 28 (67) | |
| No smoking | 7 (6) | 5 (5) | 3 (7) | |
| Comorbidity, n (%) | ||||
| Diabetes | 29 (25) | 20 (27) | 9 (20) | .423 |
| COPD | 21 (18) | 15 (20) | 6 (14) | .362 |
| Chronic kidney diseases | 14 (12) | 9 (12) | 5 (11) | .897 |
| Cardiovascular diseases | 66 (56) | 42 (57) | 24 (55) | .815 |
| Other previous cancer | 18 (15) | 11 (15) | 7 (16) | .879 |
| Type of Lung Cancer (no. 117), n (%) | ||||
| Adenocarcinoma | 64 (55) | 36 (49) | 28 (65) | .084 |
| Squamous Cells Carcinoma | 53 (45) | 38 (51) | 15 (35) | |
| Lung Cancer Stage at the Time of ICIs Therapy, n (%) | ||||
| IVA | 64 (54) | 48 (67) | 16 (36) | .001 |
| IVB | 52 (45) | 24 (33) | 28 (64) | |
| Median number of metastasis, n (IQR) | 3 (2–4) | 3 (2–3) | 3 (2–4) | .030 |
| Pts with CNS metastasis, n (%) | 15 (13) | 4 (5) | 11 (25) | .002 |
| Pts with liver metastasis, n (%) | 12 (10) | 5 (7) | 7 (16) | .118 |
| Pts with adrenal metastasis, n (%) | 14 (12) | 6 (8) | 8 (18) | .108 |
| Pts with bone metastasis, n (%) | 21 (18) | 13 (18) | 8 (18) | .959 |
| ECOG Performance Status | ||||
| 0 | 16 (13) | 12 (16) | 4 (9) | .096 |
| 1 | 83 (70) | 54 (73) | 29 (66) | |
| 2 | 19 (17) | 8 (11) | 11 (25) | |
| Median overall survival from 1 dose of ICI (IQR), months | 6 (3–11) | 6 (3–12) | 5 (2–9) | .084 |
| Immune checkpoint inhibitor line of treatment, n (%) | ||||
| First line | 30 (25) | 0 | 30 (68) | <.001 |
| Second line or more | 88 (75) | 74 (100) | 14 (32) | |
| Median number of ICI doses, n (IQR) | 9 (7–11) | 10 (7–13) | 6 (4–8) | .023 |
| Previous surgery, n (%) | 17 (14) | 11 (14) | 6 (13) | .854 |
| Radiotherapy, n (%) | 39 (34) | 25 (35) | 14 (32) | .748 |
Abbreviations: CNS, central nervous system; COPD, chronic obstructive pulmonary disease; ECOG, Eastern Cooperative Oncology Group; ICI, immune checkpoint inhibitors; IQR, interquartile range; Nr, number; Pts, patients; yr, years.
NOTE: Boldface means statistically significant (P < .05).
Overall, the main differences between the 2 groups were the number of patients with the central nervous system (CNS) metastasis (5% vs 25% in the nivolumab vs pembrolizumab group, respectively; P = .002) and the median number of ICI doses (10 [IQR, 7–13] vs 6 [IQR, 4–8] in the nivolumab vs pembrolizumab group, respectively; P = .023).
Occurrence of Infections and Immune-Related Adverse Events During Immune Checkpoint Inhibitors Treatment
During the ICI treatment, a total of 72 (61%) patients were referred to the hospital at least once for adverse events and underwent laboratory or microbiological investigations as well as radiology imaging according to the physician judgment. In 61 patients (52%), a diagnosis of IrAEs was posed without a significant difference of incidence between the 2 groups, although the need for a corticosteroid treatment was higher in the pembrolizumab group (55% vs 30% in the nivolumab group, respectively; P = .008) (Table 2).
Table 2.
Adverse Events During ICI Treatment
| Adverse Events | Total (No. 118) | Nivolumab (No. 74) | Pembrolizumab (No. 44) | P Value |
|---|---|---|---|---|
| At least 1 adverse event during ICI, n (%) | 72 (61) | 42 (57) | 30 (68) | .218 |
| Occurrence of immune adverse event, n (%) | 61 (52) | 35 (47) | 26 (59) | .215 |
| Steroid treatment during ICI therapy, n (%) | 46 (39) | 22 (30) | 24 (55) | .008 |
| A least 1 infection, n (%), cells/µL | (no. 27) | (no. 16) | (no. 12) | |
| Absolute neutrophils count at infection | 7.768 (6.317–11.037) | 7.276 (5.191–11.037) | 9.643 (7091–11.865) | .178 |
| Absolute lymphocytes count at infection | 1.440 (713–3.101) | 1.418 (732–2721) | 1.841 (620–3.141) | .926 |
| At least 1 infection, n (%) | 28 (24) | 16 (22) | 12 (27) | .485 |
| Source of Infection, n (%) | ||||
| Pulmonary | 19 (68) | 11 (69) | 8 (66) | .178 |
| Skin and soft tissue | 2 (7) | 0 | 2 (17) | |
| Urinary tract | 2 (7) | 2 (12) | 0 | |
| Gastrointestinal | 2 (7) | 2 (12) | 0 | |
| Primary BSI/fever of unknown origin | 3 (12) | 1 (6) | 2 (17) | |
| More than 1 infectious eventa during treatment, n (%) | 14 (12) | 7 (9) | 7 (16) | .379 |
| Death due to an infection, n (%) | 9 (8) | 2 (3) | 7 (16) | .008 |
Abbreviations: BSI, bloodstream infection; ICI, immune checkpoint inhibitors.
aAll recurrent infections were pneumonia: 8 patients had an additional episode of pneumonia; 5 patients had 2 episodes of pneumonia; and 1 patient presented an episode of fever and cough healed with antibiotic treatment.
NOTE: Boldface means statistically significant (P < .05).
In 28 cases (24%), a diagnosis of infection was posed and the antimicrobial treatment was initiated according to ID consultation. A total of 14 (12%) of patients experienced more than 1 infective episode. The type of ICI was not associated with an increased risk of infection in this cohort (Table 2). Of note, among the 28 patients who experienced at least 1 infection, 19 (68%) vs 9 (32%), respectively (P = .050), also experienced previous IrAEs.
Considering the first episode, the most common infection recorded was a pneumonia (68% of cases), whereas other sites of infection occurred in less than 15% of patients. In contrast, all recurrent episodes of infection involved the lower respiratory tract (Table 2). Crude mortality for infections occurred in 3 of 28 patients (11%) for the first episode, 4 of 12 patients (33%) for the second episode, and 2 of 5 (40%) patients for the third episode.
Etiology of Infections
All responsible pathogens of infections were recorded when available on medical records. In Table 3, all infections occurred during ICI treatment and all responsible organisms are shown. It is notable that no opportunistic pathogen was detected in this cohort.
Table 3.
Microbiological Diagnostic Rate Compared With the Total Number of Infectious Episodes and Type of Microorganisms
| First Episode of Infection (9/28) | ||
|---|---|---|
| Pneumonia (6/19) | ||
| Pseudomonas aeruginosa (x2) | Klebsiella pneumoniae (x2) | Haemophilus influenzae (x2) |
| Urinary Tract Infections (1/2) | ||
| Escherichia coli (x1) | ||
| Primary Bloodstream infection (1/1) | ||
| Candida albicans (x1) | ||
| Skin and Soft Tissue Infections (1/2) | ||
| MSSA (x1) | ||
| Second Episode of Infection (4/12) | ||
| Pneumonia (4/12) | ||
| MRSA (x1) | P aeruginosa (x1) | K pneumoniae (x1) |
| H influenzae (x1) | ||
| Third Episode of Infection (1/5) | ||
| Pneumonia (1/5) | ||
| P aeruginosa (x1) |
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensible S aureus.
NOTE: Diagnostic rate is expressed between parenthesis.
Predictors of All-Cause Mortality
To evaluate the predictors of mortality in our cohort, a univariable Cox regression and then a multivariable stepwise Cox regression were performed (Table 4). It is notable that, after adjusting for age, sex, comorbidity, type, stage of cancer and treatment received, ECOG performance status, and occurrence of adverse events (including infections), the variable independently associated with increased risk of mortality was a higher ECOG performance status at baseline (ECOG = 1: adjusted hazard ratio [aHR] = 2.72, 95% confidence interval [CI] = 1.48–5.00, P = .001; ECOG = 2: aHR = 4.80, 95% CI = 2.16–10.66, P < .001), whereas the multidisciplinary approach to concurrent infections was independently associated with a reduced risk of mortality (aHR = 0.50, 95% CI = 0.30–0.83, P = .008). Moreover, patients aged >75 years (only 6 patients) seemed to have a prolonged survival.
Table 4.
Independent Risk Factors for All-Cause Mortality by Cox Regression Analysis
| Univariable Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| Risk Factors | HR | 95% CI | P Value | aHR | 95% CI | P Value |
| Age Group | ||||||
| <65 years (no. 63) | 1 | 1 | ||||
| Between 65 and 75 years (no. 49) | 0.77 | 0.51–1.14 | .199 | 0.82 | 0.50–1.36 | .455 |
| >75 years (no. 6) | 0.26 | 0.09–0.74 | .012 | 0.21 | 0.07–0.64 | .006 |
| Female sex (no. 25) | 1.85 | 1.15–2.96 | .010 | 1.62 | 0.93–2.81 | .085 |
| At least 1 metabolic comorbidity (no. 93) | 0.83 | 0.52–1.32 | .432 | / | ||
| Previous Cancer (no. 18) | 0.89 | 0.52–1.51 | .670 | / | ||
| Type of Lung Cancer (no. 117) | ||||||
| Adenocarcinoma (no. 64) | 1 | 1 | ||||
| Squamous cells carcinoma (no. 53) | 0.87 | 0.59–1.28 | .493 | 0.93 | 0.61–1.43 | .775 |
| Type of ICI Treatment | ||||||
| Nivolumab (no. 74) | 1 | 1 | ||||
| Pembrolizumab (no. 44) | 1.43 | 0.96–2.14 | .076 | 0.71 | 0.32–1.59 | .416 |
| Lung Cancer Stage at the Time of ICIs Therapy | ||||||
| IVA (no. 64) | 1 | / | ||||
| IVB (no. 52) | 1.37 | 0.93–2.02 | .107 | / | ||
| Pts with CNS metastasis (no. 15) | 2.25 | 1.21–4.17 | .010 | 1.87 | 0.88–3.95 | .100 |
| Pts with liver metastasis (no. 12) | 0.86 | 0.46–1.61 | .650 | / | ||
| ECOG Performance Status | ||||||
| 0 (no. 16) | 1 | 1 | ||||
| 1 (no. 83) | 1.91 | 1.11–3.29 | .019 | 2.72 | 1.48–5.00 | .001 |
| 2 (no. 19) | 3.32 | 1.61–6.83 | .001 | 4.80 | 2.16–10.66 | <.001 |
| Immune Checkpoint Inhibitor Line of Treatment | ||||||
| First line (no. 30) | 1 | 1 | ||||
| Second line or more (no. 88) | 0.67 | 0.43–1.04 | .076 | 0.73 | 0.32–1.66 | .462 |
| Absolute neutrophils count at infection (no. 27) | 1.00 | 0.99 – 1.00 | .112 | / | ||
| Absolute lymphocytes count at infection (no. 27) | 0.99 | 0.99 – 1.00 | .426 | / | ||
| At least 1 adverse event during ICI (no. 72) | 0.88 | 0.60–1.30 | .540 | / | ||
| Occurrence of immune adverse event (no. 61) | 0.87 | 0.59–1.28 | .496 | / | ||
| Steroid treatment during ICI therapy (no. 46) | 1.20 | 0.81–1.77 | .356 | / | ||
| Previous surgery (no. 17) | 0.85 | 0.50–1.46 | .573 | / | ||
| Radiotherapy (no. 39) | 1.20 | 0.80–1.80 | .357 | / | ||
| Multidisciplinary management and treatment of infections during ICI (no. 28) | 0.64 | 0.40–1.00 | .055 | 0.50 | 0.30–0.83 | .008 |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; ICI, immune checkpoint inhibitors; pts, patients.
NOTE: Boldface means statistically significant (P < .05).
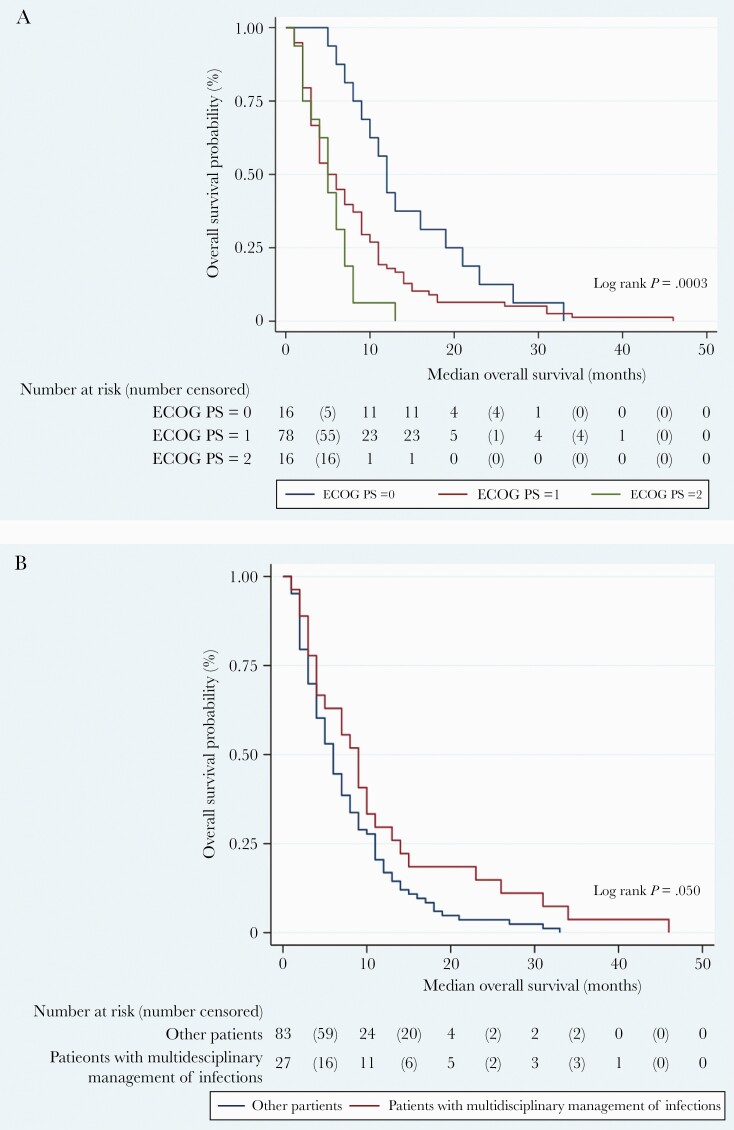
Finally, a Kaplan-Meier survival analysis was performed for variable of interest. As shown in Figure 1a, patients with worst ECOG performance status had a significantly shorter overall survival probability (log rank P = .003), whereas patients who underwent a multidisciplinary evaluation for concurrent infections demonstrated a trend towards a prolonged median overall survival (log rank P = .050) (Figure 1b).
Figure 1.
Time until death according to Eastern Cooperative Oncology Group Performance Status (ECOG) performance status (PS) (a) and infections management (b).
DISCUSSION
To the best of our knowledge, this is one of the largest cohorts of patients affected by advanced NSCLC treated with ICIs in which concurrent infections were recorded and described; furthermore, this is the first study to assess the efficacy of a collaborative approach between oncologists and ID specialists in diagnosis and management of adverse events of this population. Indeed, one of the main challenges for clinicians involved in the care of these patients is to distinguish a probable “immune-related” adverse event from a concurrent infection, because signs and symptoms of different organ involvement are often overlapping and a deeply personalized diagnostic algorithm is of crucial importance [13].
It is notable tthat the occurrence of adverse events during ICIs is far from unusual: more than half of patients included in this study experienced at least 1 adverse event, and approximately one quarter of subjects suffered from an infection. In addition, it should be considered that only moderate to severe infectious diseases that required an access to care (as outpatients or through hospitalization) were recorded in this work; therefore, the overall incidence of infectious events has been probably underestimated. Because we excluded analysis of all mild infections in this cohort, it may explain why we observed a relatively higher crude infection-related mortality rate.
However, larger studies that investigate the infection risk and possible diagnostic or prophylactic strategies for these specific populations are lacking. Indeed, secondary infections were predominantly due to bacterial etiologies in our series, and therapies did not differ from standard of care of other settings. Consistently with a recent work [5], no specific opportunistic pathogens or atypical presentations of common infections were recorded in our patients. On the contrary, in our cohort, a relatively high number of subjects experienced IrAEs, treated in all cases with corticosteroid therapy alone for less than 2 weeks. It is noteworthy that, in contrast to the study by Malek et al [5], all patients included in this study were exposed only to ICIs, and none underwent ICIs plus cytotoxic chemotherapy. In theory, the contemporary use of cytotoxic agents may be a reason for a hampered ICIs-mediated immune activation and, in turn, a reduced occurrence of IrAEs. This aspect is still unexplored, and further studies should be performed to confirm this observation, as well as compare the infectious risk of these different populations. This could also be a possible explanation for the absence of opportunistic infections in our cohort, unlike other recently published reports that showed the occurrence of PjP in conjunction with the use of prolonged corticosteroid cycles for IrAEs [7, 8].
The need for larger studies as well as dedicated guidelines is reinforced by the evidence that the most frequent type of IrAEs in literature is represented by pulmonary toxicity [14, 15]. On the other hand, as shown in our study, the majority of infections recorded are pneumonia, usually caused by “classic” Gram-negative rods/Enterobacteriaceae or methicillin-resistant-Staphylococcus aureus. These findings are consistent with the known susceptibility of lung cancer patients to pneumonia due to immunological and anatomical aberrations of the lower respiratory tract [16]. However, it should be noticed that in our series, an IrAE frequently preceded an infection, although this association was not statistically significant. This observation reinforces the need for additonal research on this topic.
Moreover, our study demonstrated that a collaborative approach to diagnosis and management of adverse events during the course of ICIs is noticeable. In fact, after adjusting for multiple covariables, the only modifiable factor that was protective for mortality was represented by a multidisciplinary management and treatment of infections during ICIs. In contrast, consistent with previous papers and current practice, the main predictor of mortality was a higher ECOG performance status [17].
In relation to mortality risk, a few details should be noted. First, note that nivolumab was prescribed according to guidelines as a second-line therapy, whereas pembrolizumab was also approved as a first-line therapy. Indeed, patients with CNS involvement at diagnosis, who frequently had ECOG <2, were treated with pembrolizumab as a first-line therapy. In contrast, patients with brain involvement at diagnosis who underwent first-line cytotoxic chemotherapy and failed the treatment, who fell to ECOG PS >2 due to the worsening (or increase in number) of CNS metastasis, were not eligible for nivolumab therapy. As a result, it is not surprising to find more patients with brain involvement at diagnosis in pembrolizumab group. Second, regarding the steroid therapy, a known risk for infections and mortality, in all cases it was not possible to define whether it was prescribed for CNS involvement, or IrAEs, or for both indications. Third, the higher death rate for infections in the pembrolizumab group at univariable analysis is surprising and is currently not easily explained. However, this information was not confirmed by Cox univariate and multivariate analysis; therefore, it was interpreted as a false association caused by the relatively low number of patients. Finally, and in contrast to the study by Malek et al [5], we did not find an association between neutrophil and lymphocyte counts at diagnosis of the infection and risk of mortality. All of these confounding variables should be explored in future studies, to better clarify their implication in risk of mortality of NSCLC patients with concurrent infections.
Nevertheless, considered that, in this case series, the diagnostic workup of concurrent infections was only based on clinicians’ judgment and not on a structured diagnostic algorithm for NSCLC patients; however, peculiar comorbidities and “frailty” of this population require a tailored approach. Therefore, one of the aims for our future projects is to prospectively investigate infections in these subjects in order to develop dedicated management and treatment strategies.
A major strength of this study is to show, for the first time, the efficacy of a dedicated approach to management of infections in a very frail group of patients. In addition, the study has implemented the body of evidence regarding the infectious risk of patients affected by NSCLC and treated with ICIs.
However, this study also has some limitations. First, as a retrospective study, many data are lacking; in addition, typical biases related to retrospective design of the study should be considered. Second, a control group of patients that were not collegially evaluated for possible infections at the onset of adverse events is absent, although a similar study that could confirm our results would not be ethical. Third, in some cases, second-line diagnostic procedures (such as bronchoalveolar lavage for pneumonitis or colonoscopy for persistent diarrhea) were not performed. These procedures would have probably increased our microbiologic diagnostic rate. Finally, a relatively small sample size also limited our ability to fully evaluate the impact of different ICIs on the risk of infections, IrAEs, and mortality.
CONCLUSIONS
In conclusion, in patients with advanced lung cancer treated with ICI, prompt management of concurrent infections could significantly improve overall survival. Further studies to investigate potential risk factors for infections, as well as appropriate management strategies and preventive measures in this setting, are warranted.
Acknowledgments
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Presented in part: 2020 World Conference of Lung Cancer Singapore, January 28–30, 2021, Virtual Meeting (https://wclc2020.iaslc.org/).
References
- 1. Michot JM, Bigenwald C, Champiat S, et al. . Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 2016; 54:139–48. [DOI] [PubMed] [Google Scholar]
- 2. Sznol M, Postow MA, Davies MJ, et al. . Endocrine-related adverse events associated with immune checkpoint blockade and expert insights on their management. Cancer Treat Rev 2017; 58:70–6. [DOI] [PubMed] [Google Scholar]
- 3. Bavaro DF, Fiordelisi D, Angarano G, et al. . Targeted therapies for autoimmune/idiopathic nonmalignant diseases: risk and management of opportunistic infections. Expert Opin Drug Saf 2020; 19:817–42. [DOI] [PubMed] [Google Scholar]
- 4. Fujita K, Kim YH, Kanai O, et al. . Emerging concerns of infectious diseases in lung cancer patients receiving immune checkpoint inhibitor therapy. Respir Med 2019; 146:66–70. [DOI] [PubMed] [Google Scholar]
- 5. Malek A, Khalil M, Hachem R, et al. . Impact of checkpoint inhibitor immunotherapy primarily pembrolizumab on infection risk in patients with advanced lung cancer: a comparative retrospective cohort study. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa802. [DOI] [PubMed] [Google Scholar]
- 6. Langan EA, Graetz V, Allerheiligen J, et al. . Immune checkpoint inhibitors and tuberculosis: an old disease in a new context. Lancet Oncol 2020; 21:e55–65. [DOI] [PubMed] [Google Scholar]
- 7. Sadek M, Loizidou A, Drowart A, et al. . Pneumocystis infection in two patients treated with both immune checkpoint inhibitor and corticoids. J Immunother Precis Oncol 2020; 3:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schwarz M, Kocher F, Niedersuess-Beke D, et al. . Immunosuppression for immune checkpoint-related toxicity can cause Pneumocystis jirovecii pneumonia (PJP) in non-small-cell lung cancer (NSCLC): a report of 2 cases. Clin Lung Cancer 2019; 20:e247–50. [DOI] [PubMed] [Google Scholar]
- 9. Oken MM, Creech RH, Tormey DC, et al. . Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5:649–55. [PubMed] [Google Scholar]
- 10. Liu YH, Zang XY, Wang JC, et al. . Diagnosis and management of immune related adverse events (irAEs) in cancer immunotherapy. Biomed Pharmacother 2019; 120:109437. [DOI] [PubMed] [Google Scholar]
- 11. Del Castillo M, Romero FA, Argüello E, et al. . The spectrum of serious infections among patients receiving immune checkpoint blockade for the treatment of melanoma. Clin Infect Dis 2016; 63:1490–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Metlay JP, Waterer GW, Long AC, et al. . Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200:e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fishman JA, Hogan JI, Maus MV. Inflammatory and infectious syndromes associated with cancer immunotherapies. Clin Infect Dis 2019; 69:909–20. [DOI] [PubMed] [Google Scholar]
- 14. Naidoo J, Wang X, Woo KM, et al. . Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol 2017; 35:709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nishino M, Giobbie-Hurder A, Hatabu H, et al. . Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol 2016; 2:1607–16. [DOI] [PubMed] [Google Scholar]
- 16. Akinosoglou KS, Karkoulias K, Marangos M. Infectious complications in patients with lung cancer. Eur Rev Med Pharmacol Sci 2013; 17:8–18. [PubMed] [Google Scholar]
- 17. Jang RW, Caraiscos VB, Swami N, et al. . Simple prognostic model for patients with advanced cancer based on performance status. J Oncol Pract 2014; 10:e335–41. [DOI] [PubMed] [Google Scholar]