Figure 11.
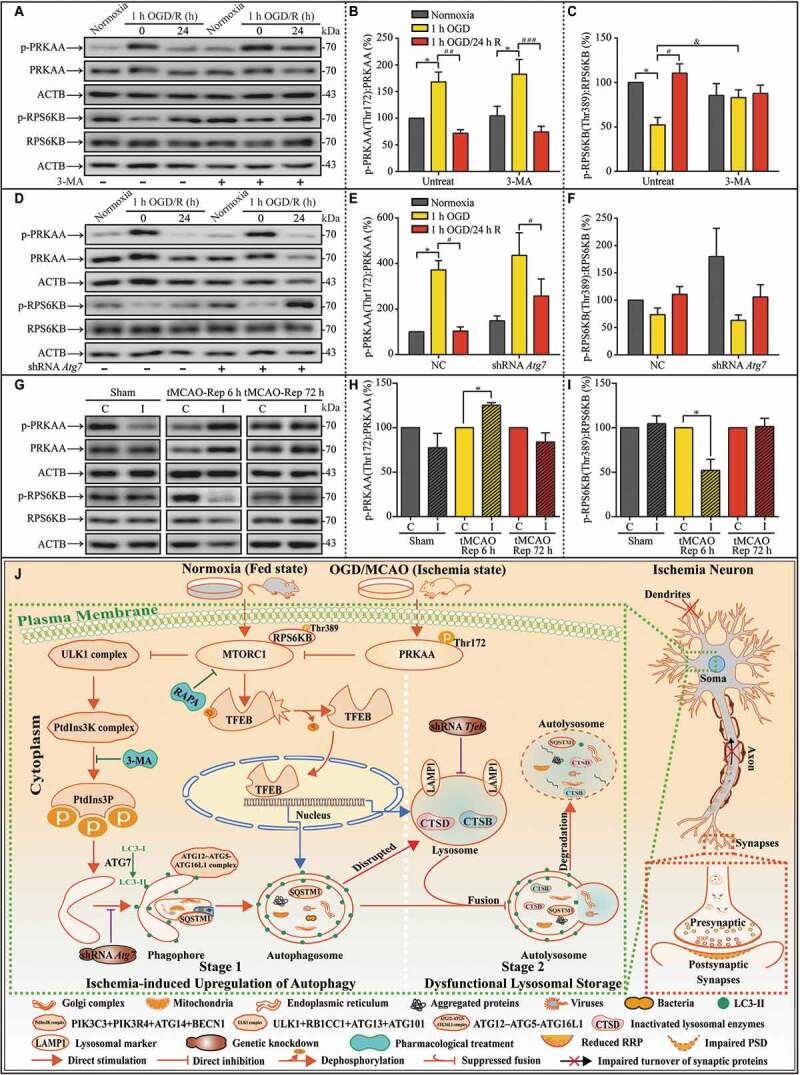
Unmatched activation levels of MTOR may contribute to lysosomal accumulation during subsequent reperfusion in in vitro and in vivo experiments. (A-C) Representative western blots and quantitative analysis of p-PRKAA, PRKAA, p-RPS6KB, and RPS6KB with or without 3-MA in cultured neurons of the indicated groups (B, n = 5; C, n = 4; *p < 0.05 vs. the indicated normoxia group; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. the indicated 1-h OGD group; &p < 0.05 vs. OGD group without 3-MA). Statistical comparisons were carried out with one-way ANOVA. (D-F) Representative western blots and quantitative analysis of p-PRKAA, PRKAA, p-RPS6KB, and RPS6KB with or without ATG7 knockdown in cultured neurons of the indicated groups (n = 4; *p < 0.05 vs. NC + the normoxia group; #p < 0.05 vs. the indicated OGD group). Statistical comparisons were carried out with one-way ANOVA. (G-I) Representative western blots and quantitative analysis of p-PRKAA, PRKAA, p-RPS6KB, and RPS6KB of MCAO mice in the indicated groups (tMCAO-Rep 6 h, n = 4; n = 3 in each of the other groups; *p < 0.05 vs. the contralateral group). Statistical comparisons were carried out with paired t-tests. Data are shown as the mean ± SEM. (J) Proposed model for the scenario of upregulated autophagy by ischemic stress, subsequent lysosomal dysfunction, and synaptic impairment in the ultra-structures. Here, we highlight the central role of neuronal autophagy in influencing lysosome functions and synaptic injuries. In this model, in the early stages of ischemia, the level of intracellular autophagy is upregulated. In the subsequent reperfusion, lysosome function regulated by MTOR signaling does not meet the requirement for the clearance of remnants of the transient autophagy burst, which leads to functional lysosomal accumulation. Finally, the impairment in turnover of synaptic proteins results in the aggravation of ischemic synaptic function damage, especially in the later stages of reperfusion. This model suggests that neuron-targeted-regulation of the lysosomal biogenesis gene MTOR may rescue dysfunctional lysosomes and improve synaptic function in later stages of reperfusion
