Figure 8.
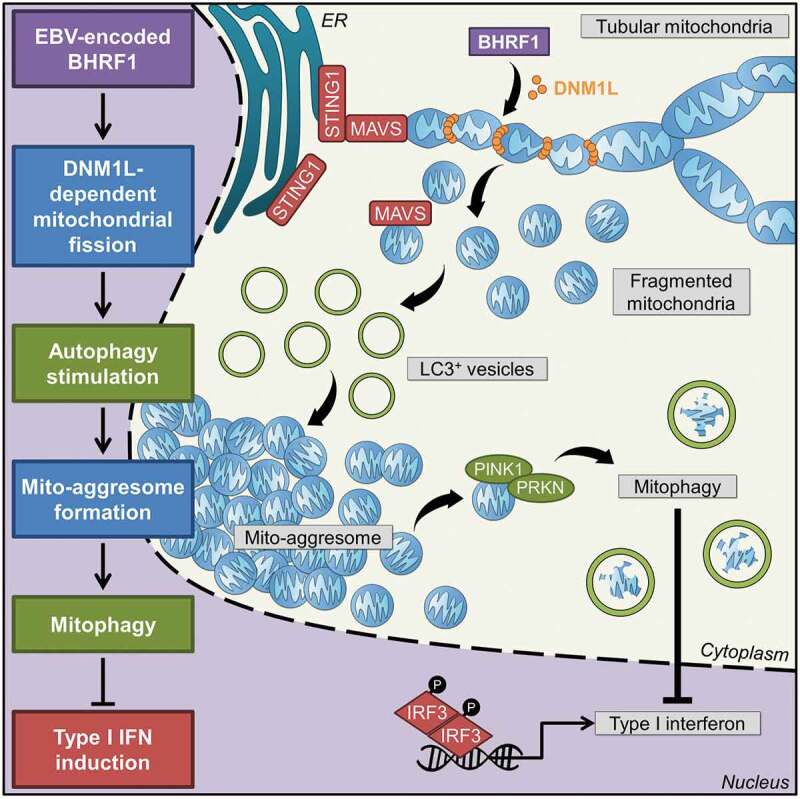
Working model. Expression of BHRF1, a BCL2 viral homolog encoded by the Epstein-Barr virus, is able to modify mitochondrial dynamics. Recruitment of a DNM1L active form upon BHRF1 expression induces mitochondrial fission. This fission is required to stimulate autophagy by BHRF1, leading to the formation of mito-aggresomes. Then, numerous mitochondria are sequestrated inside autophagosomes in order to be degraded by BHRF1-induced mitophagy. The innate immune signaling adaptors MAVS and STING1 are located at the mitochondria and at the ER, respectively. They can interact together and play a pivotal role in the antiviral response by inducing type I interferons. BHRF1 modulates the mitochondrial fate by the sequestration of mitochondria in autophagic vacuoles and mitophagy and finally leads to inhibition of type I IFN induction via both MAVS and STING1 pathways
