Fig. 2.
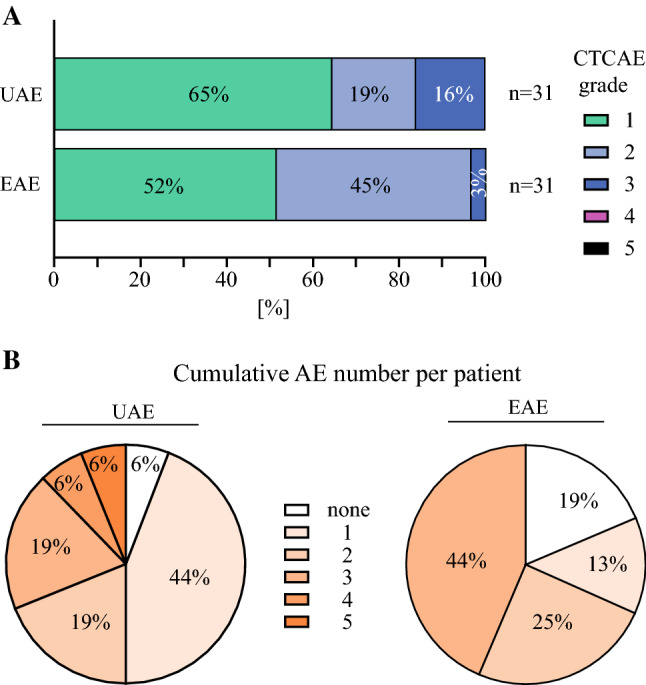
Adverse event (AE) overview in the mITT group. a Proportional distribution (%) of AEs classified according to National Cancer Institute CTCAE v.4.0, categorized as EAEs (bottom column) or UAEs (top column) [please see the Methods section (Assessment of Endpoints) for respective details]. b Proportional distribution (%) of patients experiencing (n) cumulative UAEs (left)/EAEs (right) over 30 days post-interventional follow-up. mITT modified intention-to-treat group, UAEs unexpected adverse events, EAEs expected adverse events, CTCAE Common Terminology Criteria for Adverse Events, AE adverse event
