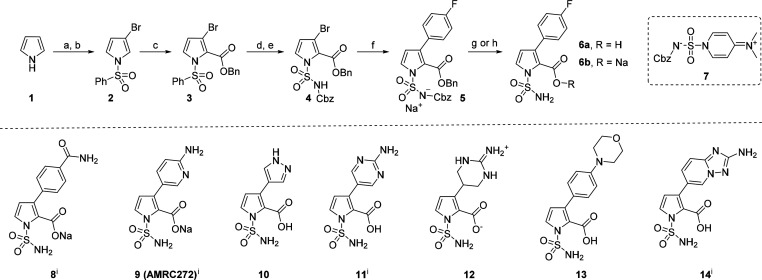
Scheme 1. Synthesis of 6a and NSPCs 8-14.
(a) NaH, then PhSO2Cl, DMF, 0 °C, 2 h; (b) Br2, AcOH, reflux, 1 h, 54% yield over two steps; (c) i-Pr2NLi, then CbzCl, THF, −78 to 0 °C; (d) 1 M TBAF, THF, rt, 2 h, 63% over two steps; (e) NaH, then 7, THF, 0 °C to reflux, 4 h, 69%; (f) 4-FC6H4B(OH)2, 5% Pd(dppf)Cl2, Na2CO3, dioxane: H2O 2:1, MW, 100 °C, 3 h, 70%; (g) 1 M aq HCl, then 10% Pd/C, H2 atmosphere, MeOH, rt, overnight, 69%; (h) 10% Pd/C, H2 atmosphere, MeOH, rt, overnight, 93%; (i) structure previously disclosed.