Abstract
SARS-CoV-2 has caused up to 127 million cases of COVID-19. Approximately 5% of COVID-19 patients develop severe illness, and approximately 40% of those with severe illness eventually die, corresponding to more than 2.78 million people. The pathological characteristics of COVID-19 resemble typical sepsis, and severe COVID-19 has been identified as viral sepsis. Progress in sepsis research is important for improving the clinical care of these patients. Recent advances in understanding the pathogenesis of sepsis have led to the view that an uncontrolled inflammatory response and oxidative stress are core factors. However, in the traditional treatment of sepsis, it is difficult to achieve a balance between the inflammation, pathogens (viruses, bacteria, and fungi), and patient tolerance, resulting in high mortality of patients with sepsis. In recent years, nanomaterials mediating reactive oxygen and nitrogen species (RONS) and the inflammatory response have shown previously unattainable therapeutic effects on sepsis. Despite these advantages, RONS and inflammatory response-based nanomaterials have yet to be extensively adopted as sepsis therapy. To the best of our knowledge, no review has yet discussed the pathogenesis of sepsis and the application of nanomaterials. To help bridge this gap, we discuss the pathogenesis of sepsis related to inflammation and the overproduction RONS, which activate pathogen-associated molecular pattern (PAMP)-pattern recognition receptor (PRR) and damage-associated molecular pattern (DAMP)-PRR signaling pathways. We also summarize the application of nanomaterials in the treatment of sepsis. As highlighted here, this strategy could synergistically improve the therapeutic efficacy against both RONS and inflammation in sepsis and may prolong survival. Current challenges and future developments for sepsis treatment are also summarized.
Keywords: Sepsis, COVID-19, RONS, Inflammation, Nanotherapy
1. Introduction
Coronavirus disease 2019 (COVID-19) broke out in late 2019 and has quickly spread across the world. In just 1 year, COVID-19 has infected more than 127 million people worldwide and caused 2.78 million deaths. Most patients with severe COVID-19 eventually develop typical septic shock, including an inflammatory cytokine storm response, cold extremities, weak peripheral pulses, oxidative stress injury, and microcirculation dysfunction [1]. Some patients exhibit obvious multiple organ dysfunction, such as heart damage, liver impairment, and kidney malfunction in addition to severe lung injury. According to the 2016 Sepsis-3 International Consensus, many critically ill COVID-19 patients satisfy the diagnostic criteria for sepsis [2]. In the latest guidelines for the treatment of COVID-19, the “surviving sepsis campaign” has been adopted as the guideline for the treatment of severely ill patients [3]. Therefore, advances in sepsis research are important for improving the clinical care of COVID-19 patients.
Sepsis is a systemic inflammatory response syndrome (SIRS) induced by an imbalance in homeostasis after exposure to a variety of injuries, including infection, trauma, and surgery, causing extremely high morbidity and mortality. Sepsis is characterized by uncontrolled inflammation and overproduction of reactive oxygen and nitrogen species (RONS), which the host immune system is unable to eliminate [[4], [5], [6]]. Subsequently, this uncontrolled inflammation and excessive RONS can damage cells and tissues, causing immune system dysfunction and eventually leading to multiple organ failure syndrome (MODS) [[7], [8], [9], [10]]. Currently, organ dysfunction caused by sepsis remains the main cause of death among hospitalized patients in intensive care units (ICUs) [8,[11], [12], [13]]. Undoubtedly, mortality from sepsis remains high, affecting more than 48.9 million people worldwide each year and is regarded as the one of the main causes of death in critically ill patients worldwide [12,14,15]. Therefore, it is necessary to investigate effective therapies to increase the survival rate of patients with sepsis.
Current therapies for treating sepsis are dominated by anti-inflammatory drugs, such as melatonin [ [16]], and traditional antioxidants, such as vitamin C [17] and N-acetylcysteine [18]. However, many studies have reported that currently available anti-inflammatory drugs and antioxidants for the treatment of sepsis are invalid and even decrease survival rates [19,20]. These drugs have many shortcomings, including low efficacy and side effects, which make it difficult to effectively reduce the inflammatory response and overproduction of RONS in sepsis [17]. Therefore, it is urgent to investigate valid strategies that focus on combating inflammation and RONS. In recent decades, the application of nanomaterials in biomedicine has been among the most fascinating areas of research, and considerable progress has been made. Nanomaterials exhibit biocompatibility, high targeting, and low toxicity, and hold immense potential for development in biomedicine [21]. For example, melanin nanoparticles that can serve as RONS scavengers to protect the ischemic brain from RONS-induced damage in a rat model of ischemic stroke have been developed [22]. Similarly, the application of nanomaterials in the treatment of sepsis has received much attention with great prospects.
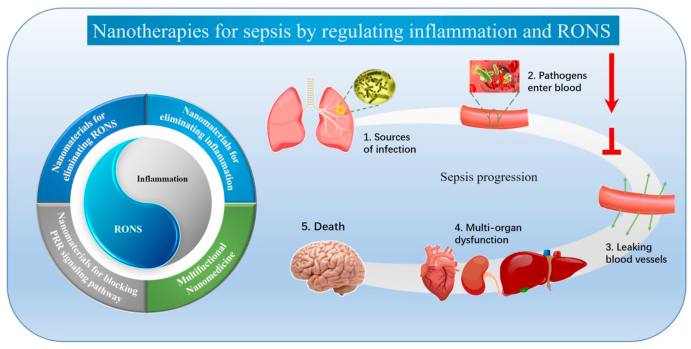
The size of nanomaterials is between that of biomolecules and cells. In theory, careful design of nanomaterials can achieve regulation of cell state and function. Therefore, the use of nanomaterials for sepsis treatment has received tremendous interest in the past two decades. Advances in nanotechnology have also made it possible to engineer complex nanostructures with unique physical properties and surface chemistry, with many potential merits compared to traditional therapeutic agents. In this review, we aim to provide a detailed account of sepsis pathology and nanotherapies for regulating inflammation and RONS in sepsis. We start with an introduction to sepsis pathology to elucidate the underlying mechanisms involved in sepsis progression, which mainly include the vicious circle of inflammation and RONS mediated by the pathogen-associated molecular pattern (PAMP)/damage-associated molecular pattern (DAMP)-pattern recognition receptor (PRR) signaling pathways, damage and dysfunction of vascular endothelial cells and immune cells caused by inflammation and RONS, and the mechanism of vascular leakage and multiple organ damage. We then highlight the progress in nanotherapies for regulating inflammation and RONS in sepsis (Table 1), which can be divided into four categories: nanomaterials blocking the PRR signaling pathways mediated by PAMPs/DAMPs, nanomaterials eliminating RONS, nanomaterials eliminating inflammation, and multifunctional nanomedicine (Fig. 1, Table 1). Finally, we discuss strategies for further improving inflammation and RONS in sepsis and the impact of treating sepsis with nanotherapies. Throughout our discussion, we provide perspectives regarding the challenges and future directions of sepsis treatment.
Table 1.
Nanocomposites for sepsis therapy.
| Category | Nanomaterials | Classification of nanomaterials | Cargo | Biological target | Efficacy | Advantages of nanomaterials | Defects of nanomaterials | Refs |
|---|---|---|---|---|---|---|---|---|
| Nanomaterials Blocking PRR Signaling Pathways | SAuNCs | Au | / | LPS | Decreased inflammatory cytokines levels and prolonged survival time in sepsis mice | Effectively inhibited the activation of TLR4 signaling pathway | Potential toxicity, including specific toxicity such as gastrointestinal perforation and bleeding, to the GI route [23]. | [24] |
| MΦ-NPs | PLGA | / | LPS | Presented a low level of inflammatory cytokines and death rate in LPS-induced sepsis mice | Inherited the biological characteristics of macrophage cell membrane | The manufacturing process of biomimetic nanoparticle is complicated and expensive. | [25] | |
| Fe3O4 nanoparticles coated with macrophage membrane | Fe3O4 | / | LPS | Decreased ROS and inflammatory cytokines levels; increased SOD activity and survival time in sepsis mice | High selectivity and affinity to PAMPs and PRRs; increased stability | Magnetic iron oxide nanoparticles are toxic to many organs [26] | [27] | |
| MSN-PEI | Silica | / | cfDNA | Scavenging cfDNA; decreasing inflammatory cytokines levels; improving tissue and survival rate in sepsis mice | Exhibited high accumulation in inflamed tissue with negligible toxicity | Nanoparticles with higher charge density have toxicity to liver and kidney. | [28] | |
| TD-NT | High polymer materials | / | PAMPs/DAMPs | Decreased inflammatory cytokines levels and improved tissue damage in sepsis mice | Captured DAMPs and PAMPs simultaneously | / | [29] | |
| Nanomaterials for RONS Elimination | CeNPs | Ceria | / | Antioxidant enzyme | Decreased RONS and inflammatory cytokines levels; improved survival rate and protected liver from sepsis-induced injury | / | Large doses of ceria oxide nanoparticles may induce liver injury and oxidative stress. | [30] |
| / | Improved diaphragmatic inflammation and function | / | [31] | |||||
| / | Inhibited inflammatory cytokines secretion; alleviated hepatic damage; increased survival rate | / | [32] | |||||
| CZ-NPs | Cerium and Zirconia | / | Antioxidant enzyme | Exhibited a decreased RONS levels and an improvement of tissues damage in sepsis mice | Exhibiting a stronger antioxidant activity | Large doses of ceria oxide nanoparticles may induce liver injury and oxidative stress. | [33] | |
| 6-AHA-CeNPs | 6-AHA-modified cerium oxide | / | Antioxidant enzyme | Exerted antioxidant and anti-inflammatory capacity; increased survival rates; improved lung and liver tissues damage | Increasing stability and bioavailability of ceria nanozyme; decreasing toxicity | Large doses of ceria oxide nanoparticles may induce liver injury and oxidative stress. | [34] | |
| Co/PMCS | Co and porphyrin | / | Antioxidant enzyme | Presented a low level of RONS and an improvement of DNA damage, lipid peroxidation and inflammatory cytokines release | Exhibiting a stronger antioxidant activity | / | [6] | |
| 2D-TMD | WS2/MoSe2/WSe2 | / | / | Exerting antioxidant and anti-inflammatory effects; alleviating multiple tissues damage; increased survival rate | Scavenging various RONS | / | [35] | |
| Nanoparticles loaded with melatonin | PEG-b-PPS | Melatonin | / | Ameliorated inflammatory and oxidative injury | Increasing bioavailability and targeting of melatonin | / | [36] | |
| Inflammation Regulation | APS nanoparticles | Chitosan/tripolyphosphate | APS | / | Inhibited inflammatory cytokines secretion; improved the structure and function of cardiac tissue | / | / | [37] |
| Nanoparticles loaded with curcumin | Solid lipid | Curcumin | / | Exerted anti-inflammatory activity; increased survival rate | Protecting curcumin from chemical degradation; controlling sustained release; increasing bioavailability | Unpredictable gelation tendency and inherent low incorporation rates [38] | [39] | |
| Macrophage-derived NVs | Leukosomes | / | 94 nm | Alleviated inflammation; prolonged survival time | Retaining the characteristics of source cells | / | [40] | |
| MSC-derived NVs | MSCs | / | / | Exerting anti-inflammatory effects | Retaining the characteristics of source cells; selective in vivo distribution | / | [41] | |
| AuNPs | Au | / | / | Induced M2 macrophage polarization and exerted anti-inflammatory effects | / | / | [42] | |
| SPIONs | Fe2O3 | / | / | Alleviated inflammation and tissue damage in septic mice | Low toxicity and excellent biocompatibility | Oxidative stress and disturbance in iron homeostasis [43]. | [44] | |
| Polymer nanoparticles | PLGAHi-PEMA, PLGAHi-PVA, PLGALo-PEMA, PLGALo-PVA, PLA-PEMA, PLA-PVA | / | / | Exerted strong anti-inflammatory effects | / | / | [45] | |
| TFMG | sFn | / | Endothelial cells | Alleviated vascular inflammatory response including decreased the vascular permeability, CAM expression, as well as inhibited the migration of circulating leukocyte to vascular endothelium.; increased survival rate; improved tissues damage | Avoiding the risk of bleeding; increasing efficacy | / | [46] | |
| Multifunctional Nanomedicine | DOX-hyd-BSA NPs | BSA nanoparticles | DOX | Neutrophils | Alleviated inflammatory injury by inducing neutrophils apoptosis and inhibiting inflammatory cytokines secretion; increasing survival rate | Increasing targeting and cell uptake | / | [47] |
| SQAd/VitE NPs | SQ nanoparticles | Ad/VitE | / | Exerted strong anti-inflammatory effects; improved survival rate, increased systolic blood pressure and decreased MSS in sepsis mice | Prolonging circulation time; reducing toxicity; increasing targeting and selectively; achieving anti-inflammatory and antioxidation combined therapy | / | [48] | |
| Nanoparticles loaded with CLP and TPCA-1 | Biotin-PEG-b-PAE(-g-PEG-b-DSPE)-b-PEG-Biotin | CLP/TPCA-1 | / | Suppressed the peritoneal infection; protected vascular integrity; inhibited inflammatory response in septic mice | Increasing targeting and selectively; achieving the anti-inflammatory and antibiotic combined therapy; increasing the bioavailability of the cargo | / | [49] | |
| Nanoparticles loaded with SFX and TAC | PLGA | SFX/TAC | / | Decreased the secretion of proinflammatory cytokines; inhibited bacterial proliferation; improved survival rates of sepsis mice | Possessing excellent bioavailability, low systemic toxicity, and hemolysis ratio | / | [50] |
Fig. 1.
The scope and focus of this article. The pathogens caused by infection will enter the blood and excessively accumulate in the lesion foci, causing massive inflammation and releasing RONS through activation of the innate immune system. This will lead to blood vessel leakage and further organ dysfunction, and even death. Therefore, blocking activation of the immune system and eliminating the inflammation and RONS is extremely important in treating sepsis. Nanomaterials provide a breakthrough for the treatment of sepsis and can be divided into the following categories: blockade of PRR signaling pathways, nanomaterials for RONS elimination, nanomaterials for eliminating inflammation, and multifunctional nanomedicine.
2. RONS production and pathogenesis of sepsis
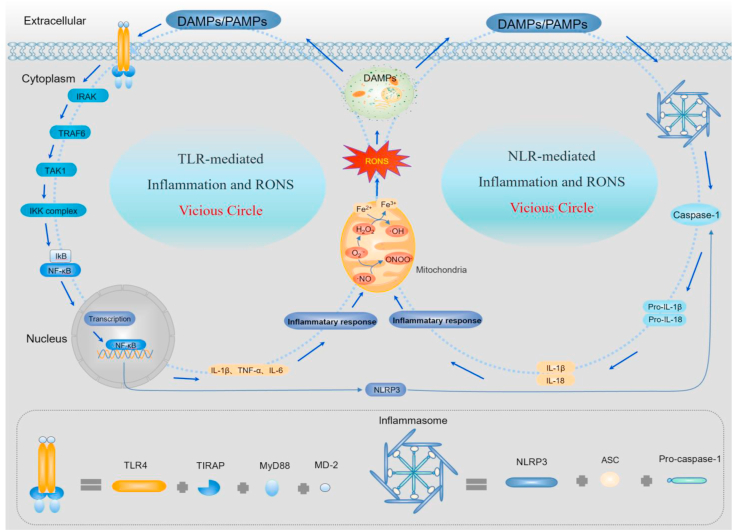
The pathogenesis of sepsis has not been well investigated, but uncontrollable inflammation and excessive RONS are thought to be two key factors that promote the progression of sepsis [51]. Therefore, an in-depth understanding of the mechanisms involved in immune response-induced inflammation and inflammation-induced RONS production is crucial for exploring sepsis pathogenesis and effective means of therapy [52]. In sepsis patients, the activated innate immune system is mediated by PRRs expressed on immune cells (e.g., macrophages and neutrophils) and non-immune cells (e.g., vascular endothelial cells) [8,53]. PRRs include Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), retinoic acid-inducible gene-like receptors (RLRs), and C-type lectin receptors (CLRs) [51,54]. In sepsis, inflammation is mainly mediated by the TLR and NLR signaling pathways [55] (Fig. 2).
Fig. 2.
Pathological mechanism of sepsis. Under septic conditions, the invasion of immunogens (PAMPs and DAMPs) can over-activate the innate immune system through TLR- and NLR-mediated signaling pathways, causing massive inflammatory cytokine release. These inflammatory cytokines damage the mitochondrial respiratory chain, triggering RONS overproduction. Importantly, because RONS can induce cell apoptosis and reactivate PRR signaling pathways by inducing DAMP formation, it creates a vicious cycle between inflammation and RONS. Thus, inflammation and RONS are the two core factors in the pathological mechanism of sepsis.
The TLR-mediated signaling pathway can be overactivated by PAMPs or DAMPs and lead to the overproduction of pro-inflammatory cytokines, such as interleukin-1β (IL-1β), tumor necrosis factor (TNF-α), and IL-6, causing mitochondrial dysfunction, such as an impaired respiratory chain, decreased ATP production, mitochondrial matrix swelling, and collapse of the membrane potential [[56], [57], [58], [59]]. Eventually, a large amount of O2·- is produced by electron leakage at the ubiquinone sites of mitochondrial respiratory chains complex I and complex III [55,[60], [61], [62]]. Excessive O2·- can be catalyzed by superoxide dismutase (SOD) to H2O2, and the unstable oxygen bond of H2O2 can react with Fe2+ or another metal ion to form the powerful oxidant OH· [63,64]. Under inflammation, inducible nitric oxide synthase (iNOS) can be over-activated by pro-inflammatory cytokines [65] and cause excessive NO· generation. Importantly, excessive O2·- can react with NO· to generate highly reactive ONOO−. Excess RONS can cause cell apoptosis, necrosis, and lysis, which release high quantities of DAMPs [66]. These DAMPs are further recognized by TLRs and prompt inflammatory cytokine and RONS production, forming a vicious circle [51]. NLRs are another important type of receptor that induces excessive inflammation and RONS during sepsis. Under septic conditions, NLRP can form a multiprotein complex or inflammasome, which assembles with the adaptor protein apoptosis-associated speck-like containing a CARD domain (ASC) and caspase-1 [[67], [68], [69]]. After the NLRP inflammasome is activated, it can promote caspase-1-induced cleavage and maturation of pro-IL-1β and pro-IL-18 to form IL-1β and IL-18, which induce mitochondrial damage, such as TLR signals, and accelerated generation of highly reactive RONS [70]. Importantly, excessive RONS then induce the assembly and activation of NLRP3 inflammasomes.
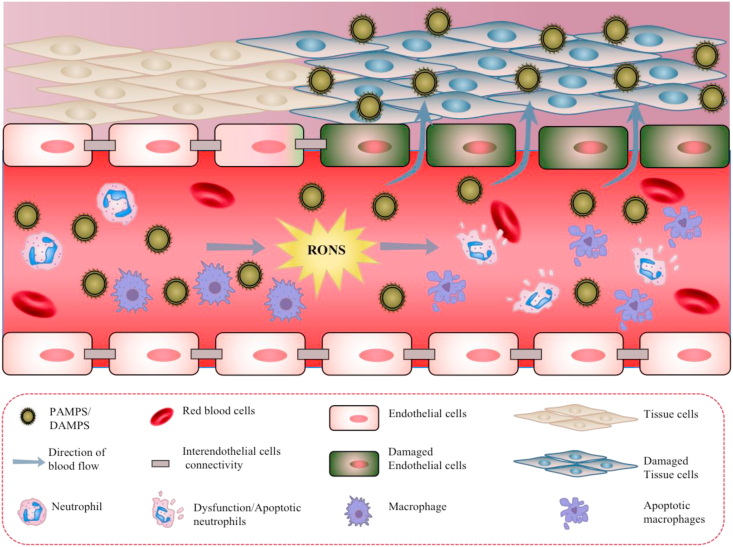
Excessive inflammation and RONS cause damage to proteins, lipids, and nucleic acids, which can cause massive immune cell and endothelial cell injury and apoptosis, ultimately leading to an immunosuppressive state in the body [55,71]. Excessive inflammation and RONS also impair the activity of non-immune cells, such as endothelial cells [72]. The endothelium is a selective permeable barrier between the vascular wall and blood flow and one of the first protective barriers against external invasion [73]. In sepsis, excessive inflammation and RONS will increase endothelial cell permeability, leading to the leakage of fluids and proteins through the vascular wall, decreased tissue perfusion, hypovolemia, tissue edema, and eventually multiple organ failure [51,74]. Therefore, it is crucial to control inflammation and remove excessive RONS, breaking the cycle between inflammation and RONS. Preventing immune disorders caused by the damaged immune cells and protecting the function of non-immune cells (i.e., endothelial cells) is also necessary for the treatment of sepsis (Fig. 3). Notably, damaged tissues have greatly increased vascular permeability, which also greatly increase the selective enrichment of nanomaterials in damaged tissues and organs during sepsis. As a result, nanotherapy is very promising for the treatment of sepsis. Rationally designing nanomaterials can not only block PAMPs/DAMPs, but also eliminate ROS and decrease pro-inflammatory factors in damaged organs to achieve high efficiency treatment of sepsis.
Fig. 3.
In sepsis, PAMPs/DAMPs cause the overproduction of RONS, which damage immune cells (macrophages and neutrophils) and non-immune cells (endothelial cells). In addition, RONS cause increased permeability of the endothelium, leading to PAMP/DAMP leakage across the vascular wall to other non-infected tissue, damaging normal cells and tissues and causing apoptosis.
3. The correlation between COVID-19 and sepsis
Sepsis is dominantly caused by an imbalance of host to infection, which is mainly induced by bacteria, viral, fungus [75]. The characteristics of viral sepsis and bacterial sepsis are extremely resemblance at the pathophysiological level. One of the manifestations of COVID-19 is fulminant disease characterized by sepsis [76]. For example, COVID-19 can cause uncontrolled inflammatory response characterized by prominent pro-inflammatory cytokine release in patients with severe COVID-19 [77,78]. Ivan Cekerevac et al. evaluated the concentrations of RONS, as well as the activity of enzymes of the antioxidative defense system in COVID-19 patients, and confirmed that the levels of O2·-, NO, and catalase activity are differently change in the different severity of COVID-19 infection [79]. Mireille Laforge et al. postulate neutrophils generated ROS that exacerbates the host immunopathological response, resulting in more severe disease and concluded that ROS generation are positive correlation with COVID-19 disease severity [80]. In short, Sepsis caused by COVID-19 can induce the overproduction of RONS and cytokines storm. These characteristics are accordant with bacterial sepsis. It is very difficult to distinguish between viral sepsis and bacterial sepsis. Recent studies have found that the distinct of viral and bacterial sepsis can only be distinguished by a complex method of whole blood genomics [81]. The therapy of sepsis is mainly combining the supportive therapy with the interventions aimed at the root causes of pathogens. For example, the therapeutic of bacterial sepsis is the combine of antibiotic with supportive therapy, and the viral one is the unite of antiviral and supportive therapy [82]. Simple antibiotic treatment or antiviral treatment is often ineffective for sepsis. Supportive treatment is the key to restore the patient's immune system to treat sepsis. Supportive treatment is mainly aimed at the pathophysiological characteristics of the treatment, such as cytokine storm and oxidative stress damage. Therefore, the latest advances of nanotherapies for sepsis by regulating inflammatory signals and RONS are crucial referential value for the therapy of sepsis in COVID-19 patients.
4. Nanomaterials blocking PRR signaling pathways
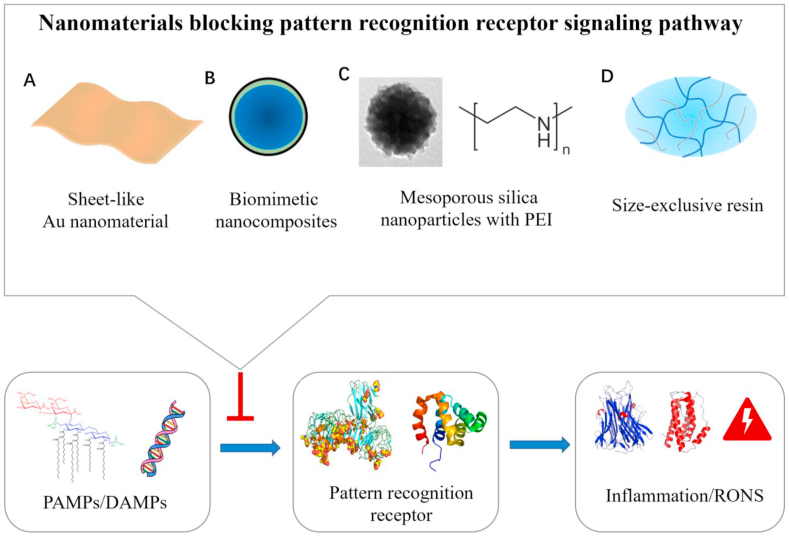
As discussed above, PAMPs and DAMPs can induce the vicious cycle between the inflammatory response and RONS through the recognition of PRRs on immune cells or non-immune cell membranes [51,83]. The binding of PAMPs/DAMPs to PRRs is the trigger that activates the vicious cycle [84]. Thus, it is an effective approach to target signaling pathways with specific nanomaterials for the treatment of sepsis (Fig. 4).
Fig. 4.
The nanomaterials including the Sheet-like Au nanomaterial (A), Biomimetic nanocomposites (B), Mesoporous silica nanoparticles with PEI(C), and Size-exclusive resin(D) blocking the binding of PAMPs/DAMPs and PRRs, thereby inhibiting the production of inflammation and RONS.
PAMPs are mainly derived from Gram-negative bacteria, Gram-positive bacteria, fungi, and viruses. Lipopolysaccharide (LPS) is the cell wall component of Gram-negative bacteria. It associates with serum LPS binding protein and binds to membrane receptor CD14 and accessory molecule MD2 to induce TLR4 signaling, producing pro-inflammatory factors and RONS. Therefore, reducing the binding capacity of LPS and TLR4-MD2 is an effective way to block the PAMP-PRR signaling pathway. The major toxic component of LPS is lipid A, and the affinity between lipid A and TLR4-MD2 receptor complexes is closely related to the molecular conformation (aggregation degree) of lipid A; the denser the aggregation degree of lipid A, the lower the affinity of LPS and TLR4-MD2. Recently, Liao et al. developed a sheet-like nanomaterial based on gold nanoclusters (SAuNCs) coated with a layer of short-chain alkanes (Fig. 4A) [24]. The short-chain alkanes on the surface of the SAuNC can bind to LPS and cause the aggregation density of lipid A to increase. As a result, SAuNC can effectively inhibit activation of the TLR4 pathway and reduce the generation of pro-inflammatory cytokines and RONS. In the LPS-induced septic mouse model, SAuNC significantly inhibits NF-κB-dependent pro-inflammatory cytokines and chemokines. Decreased cytokines can inhibit RONS production, prolong the survival of mice with sepsis.
The capture of PAMPs with specific nanoparticles is also an important method of blocking the PAMP-PRR signaling pathway [85,86]. As mentioned above, PAMPs usually include substances derived from a variety of different pathogens. Therefore, only blocking the LPS/PRR pathway cannot effectively treat sepsis caused by COVID-19. It has been extremely challenging to design nanomaterials that can directly capture a wide range of PAMPs. This bottleneck can effectively be solved using biomimetic nanocomposites, which are mainly prepared by coating specific nanomaterials with immune cell membranes. Immune cell membranes (e.g., macrophage cells) usually contain abundant TLRs, which can efficiently bind different kinds of PAMPs [[87], [88], [89]]. Furthermore, binding of PAMPs and the biomimetic nanocomposites cannot initiate activation of the PRR signaling pathway due to lack of downstream effectors, such as MyD88. A well-designed nanoparticle core can stabilize the immune cell membrane and prolong its activity in the physiological environment. For example, Thamphiwatana et al. developed a biomimetic nanoparticle consisting of a biodegradable poly (lactic-co-glycolic acid) (PLGA) nanoparticle core and macrophage-derived cell membrane (denoted MΦ-NPs) [25]. The hydrophilic surface glycans of the PLGA core can stabilize the macrophage cell membrane and improve the stability of MΦ-NPs in serum. In addition, MΦ-NPs inherit the biological characteristics of the macrophage cell membrane and can effectively combine with PAMPs. Following the treatment of septic mice, MΦ-NPs decrease the concentrations of pro-inflammatory cytokines in the blood and spleen, inhibit RONS production, and improve survival in mice with sepsis. Recently, magnetic separation technology has been applied to biomimetic nanocomposites to further improve the efficiency of capturing PAMPs [90,91]. For example, Shen et al. designed biomimetic nanocomposites composed of macrophage membrane and polyethyleneimine (PEI)-modified Fe3O4 nanoparticles (Fe3O4@MM NPs) [27]. The positively charged Fe3O4 cores can stabilize the macrophage membrane, increase the affinity for PAMPs, and achieve magnetic separation of PAMPs. Fe3O4@MM NPs effectively avoid activation of the PRR signaling pathway and reduce the secretion of TNF-α and IL-6 and other cytokines, ultimately improving the survival rate of sepsis mice.
DAMPs are endogenous molecules that can initiate and enhance the non-infectious inflammatory response [92,93]. For example, many DAMPs (e.g., cell-free DNA [cfDNA]) are released into the circulatory system after cell breakdown and necrosis in sepsis. DAMPs over-activate the TLR9-MyD88-NF-κB signaling pathway and induce the overproduction of inflammatory cytokines and RONS [92,94,95]. Therefore, blocking the DAMP-PRR signaling pathway is also an important aspect of inhibiting the host's overactive immune system. In theory, PEI has a positive charge and can effectively capture cfDNA. However, PEI also has strong cytotoxicity that cannot be ignored. The efficiency of PEI in blocking TLR9 activation and its toxicity both positively correlate with the charge density on PEI. To solve this dilemma, Dawulieti et al. prepared biodegradable mesoporous silica nanoparticles functionalized with PEI (MSN-PEI) [28]. The PEI of MSN-PEI can effectively prevent activation of the TLR9-MyD88-NF-κB signaling pathway by capturing cfDNA. Here, large-pore MSN served as the PEI carrier to reduce toxicity and maintain the capture efficiency of PEI. Accordingly, MSN-PEI administration effectively inhibited the secretion of TNF-α, IL-6, and MCP-1 in serum and the peritoneal fluid in cecal ligation and puncture (CLP)-induced septic mice. In addition, MSN-PEI alleviated multiple organ damage and markedly reversed the increase alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen, creatinine, creatine kinase, and total bilirubin levels in serum.
In sepsis patients, immune system dysfunction is largely the result of the synergistic effects of PAMPs and DAMPs [51]. Therefore, simultaneously blocking the DAMP- and PAMP-PRR pathways is more effective than blocking a single pathway [96]. In terms of molecular structure, most DAMPs and PAMPs contain hydrophobic groups and negatively charged groups. As for immune regulatory factors in the body, pro-inflammatory factors generally have a negative charge and anti-inflammatory factors a positive charge. On this basis, Shi et al. designed a linear-dendritic telodendrimer nanotrap (TD-NT) with multiple charges and hydrophobic moieties to capture PAMPs, DAMPs, and pro-inflammatory factors [29]. The TD-NTs can bind PAMPs, DAMPs, and inflammatory cytokines to reduce excessive inflammation during sepsis through interactions between multivalent electrostatic and hydrophobic domains. In addition, the molecular weight of cytokines and PAMPs/DAMPs is usually less than 30 kDa, smaller than most serum proteins (>50 kDa). The authors loaded the TD-NTs into 50-kDa hydrogel resins, which prefer capturing cytokines and PAMPs/DAMPs in serum.
5. Nanomaterials for RONS elimination
Under physiological conditions, redox homeostasis is preserved in the body by redox buffering systems. The system's delicate balance is preserved by antioxidant enzymes containing SOD, catalase, and glutathione peroxidase (GPx) [55]. In sepsis, excessive RONS far exceed the scavenging ability of antioxidant enzymes in vivo and can cause oxidative stress in the body, which in turn produces more RONS [57,97,98]. Therefore, it is necessary to rely on the administration of exogenous antioxidants to maintain the body's redox balance. However, traditional antioxidants present many shortcomings, such as severe side effects, rapid clearance, and low stability and bioavailability, which limits their clinical application. Recent studies have found that nanomaterials have great potential in the treatment of sepsis by directly removing RONS or by delivering traditional antioxidants, which not only overcome the shortcomings of traditional antioxidants, but also improve clinical efficacy [99] (Fig. 5).
Fig. 5.
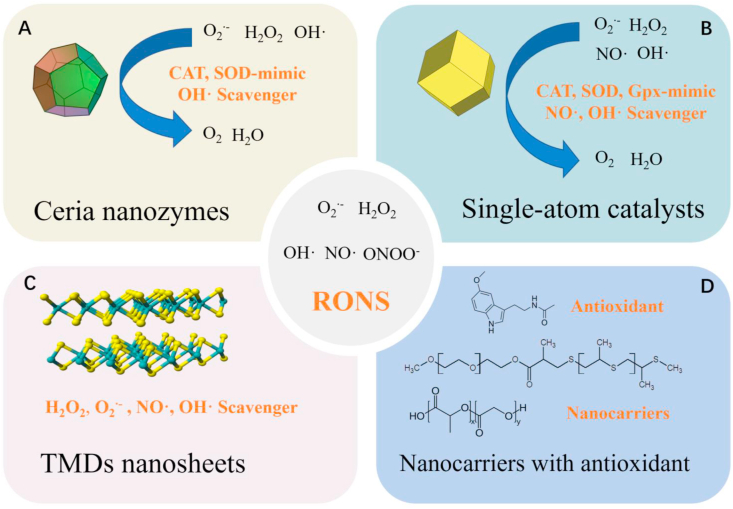
Some of nanomaterials can eliminate excessive RONS in vivo through mimicking multiple antioxidant enzyme, including Ceria nanozymes(A) and Single-atom catalysts(B). Except that, TMDs nanosheets can effectively scavenge various RONS to treat sepsis(C), as well as nanomaterial can serve as carriers delivering antioxidant to inflammation site(D).
5.1. Nanozymes and single-atom catalysts
Natural antioxidant enzymes in the body exert excellent ROS scavenging activity [100]. However, their practical application is not satisfactory for two reasons. First, natural enzymes are unstable under physiological conditions and easily degraded by proteolytic enzymes [101]. Second, these natural antioxidant enzymes usually only eliminate one type of ROS and sepsis is caused by multiple RONS. Thus, only eliminating one kind of RONS cannot achieve the desired therapeutic effect and it is necessary to find antioxidant enzyme mimics that effectively scavenge multiple RONS. Notably, some nanomaterials are inherently antioxidant, which can decrease the levels of multiple RONS in the body by mimicking antioxidant enzymes. Nanomaterials with enzyme-mimetic activity are nanozymes [[101], [102], [103]]. Due to the high catalytic activity, good stability, low manufacturing cost, and ability to mimic multiple antioxidant enzymes [102,104], nanozymes have great advantages in removing excess RONS during sepsis. Currently, cerium oxide (ceria) nanozyme and single-atom catalyst (SAC) nanozyme have been developed and exhibit excellent therapeutic effects in sepsis.
On the nanoscale, ceria has stronger catalase or SOD enzyme-like activity, and the Ce3+/Ce4+ surface ratio is closely related to the antioxidant enzyme-mimetic activity [105,106]. For example, ceria nanozyme has high Ce4+ state surface atoms that behave as catalase-mimics (Ce4+ + H2O2 + 2OH· → O2 + 2Ce3+ + H2O), whereas the ceria enzyme with high amounts of Ce3+ acts as a SOD-mimic (Ce3+ + 2H+ + O2·- → H2O2 + Ce4+) [107,108]. In addition, the ceria nanozyme can eliminate OH· through a redox reaction (Ce3+ + 2OH· → 2Ce4+ + H2O) [109]. Therefore, ceria nanozyme can be applied in the treatment of sepsis by eliminating ROS (Fig. 5A). Selvaraj et al. prepared ceria nanozymes to effectively eliminate excessive ROS in sepsis, with a distinct reduction in NO generation and iNOS expression observed in septic mice after treatment with ceria nanozymes [30]. The ceria nanozymes also decreased the expression of cyclooxygenase-2 (COX2) and the levels of pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) in macrophages from septic mice. Notably, COX-2 could induce the synthesis of prostaglandin E2 (PGE2), which stimulated pain and inflammation. The ceria nanozyme significantly improved survival and protected the liver from sepsis-induced injury. Similarly, other studies have also shown that ceria nanozymes can reduce functional sepsis-induced injury to the liver and diaphragm through their antioxidant and anti-inflammatory activities [31,32].
However, the application of ceria nanozyme is limited by its potential toxicity and low stability in sepsis. Therefore, current studies have tried to improve the antioxidant activity of ceria nanozymes, as well as reduce its potential toxicity. For example, Min Soh et al. synthesized 2-nm ceria-zirconia (CZ) nanozymes to serve as enhanced antioxidants for sepsis treatment [33]. The CZ-nanozymes have a higher Ce3+/Ce4+ ratio and faster conversion from Ce4+ to Ce3+ than ceria nanozymes due to the addition of Zr to the ceria nanozyme. Therefore, CZ-nanozymes exhibit stronger antioxidant activity for scavenging ROS than ceria nanozymes. In addition, the small size (2 nm) gives CZ-nanozymes lower toxicity than ceria nanozymes, which have a size of 3–4 nm. Consequently, CZ-nanozymes markedly reduce inflammatory injury in major tissues, including the liver, lungs, cecum, stomach, and small intestine in mice with CLP-induced sepsis. Cerium oxide nanozymes can also increase the ROS-eliminating activity and reduce toxicity to a certain extent by improving their stability under physiological conditions. Jeong et al. fabricated polyethylene glycol (PEG)ylated ceria nanozymes with 6-aminohexanoic acid (6-AHA) as a surface stabilizer [34]. The 6-AHA-PEG stabilizer increases the colloidal stability of ceria nanozymes in the physiological environment and prevents surface adsorption of the biological components, including phosphate ions and serum proteins. Notably, phosphate ions preferentially bond to surface Ce3+ or Ce4+ sites to form CePO4−-like complexes on the ceria nanozymes and prevent redox cycling between Ce3+ and Ce4+. In addition, the PEGylated formulation increases the circulating blood half-life and cellular uptake, avoiding rapid clearance and cytotoxicity. Therefore, PEGylated 6-AHA-ceria nanozymes possess excellent ROS scavenging and anti-inflammatory capacity.
The catalytic active centers of many nanozymes and natural enzymes are generally coordinated metal centers. SACs are catalysts in which the catalytic active center of the metal is embedded in a catalytic carrier with a single atomic scale. For nanozymes, the catalytic activity comes from the metal located on the surface of the nanozymes. Most of the metal atoms are buried under the surface and cannot be reached by the reactants. SACs can ensure that all metal active centers play a catalytic role and usually have higher catalytic activity and less potential cytotoxicity [110,111]. Recently, Cao et al. developed an enzyme-mimicking SAC (Co/PMCS) by atomically dispersing co-porphyrin centers in nitrogen-doped carbon-supported nanomaterials for sepsis treatment (Fig. 5B) [6]. Co/PMCS could scavenge O2·- by simulating SOD, eliminate H2O2 by simulating catalase and GPx, and remove OH· via the oxidative-reduction cycle. Furthermore, it could eliminate NO· via coordination of the co-porphyrin center. Co/PMCS significantly decreased the level of RONS and further reduced the secretion of inflammatory cytokines, such as IL-6 and TNF-α. The survival rate and damage to multiple organs in mice with sepsis also improved, which suggests that Co/PMCS can effectively protect cells from oxidative stress and inflammatory damage.
5.2. RONS nanoscavengers
In addition to nanozymes, RONS nanoscavengers also play important roles in antioxidant therapy by directly reacting with excessive RONS [112]. Recently, two-dimensional (2D) transition-metal dichalcogenide (TMD) nanosheets were proposed as novel RONS scavengers that can be applied in the treatment of sepsis [35]. Compared to conventional antioxidants, 2D-TMD nanosheets can effectively remove various RONS with low cytotoxicity, good biocompatibility, structural stability, and durability of action [113,114]. Notably, Yim et al. synthesized three kinds of 2D-TMD nanosheets (WS2, MoSe2, and WSe2) functionalized with poly(ε-caprolactone) (PCL)-b-PEG for the treatment of sepsis (Fig. 5C) [35]. The amphiphilic PCL-b-PEG contributes to the exfoliation of 2D-TMD nanosheets in water. These nanosheets undergo oxidation reactions with multiple RONS and exhibit a superior capacity for continuously removing H2O2, O2·-, OH·, and NO·. The WS2 TMD nanosheets exhibit stronger ROS scavenging capacity than the molybdenum (Mo)-based TMDs (WS2 > WSe2 > MoSe2). Accordingly, these TDMs significantly decrease RONS in inflammatory cells, suppress the secretion of inflammatory cytokines, and have no effects on anti-inflammatory cytokines. In the septic mouse model, these TDMs significantly improve survival and tissue damage in the lungs, liver, and spleen. Similarly, the WS2 nanosheet has the best therapeutic effects in sepsis treatment, which may be attributed to their long retention in the body.
5.3. Nanocarriers loaded with antioxidant
Sepsis is a disease closely related to oxidative stress. In theory, treatment of sepsis with antioxidants is a promising strategy. However, the therapeutic potential of many antioxidants (e.g., natural antioxidants, endogenous antioxidants, synthetic organic antioxidants) is restricted by factors such as solubility, stability, and low bioavailability (e.g., poor gastrointestinal absorption). Recently, some novel nanocarriers were developed for the delivery of antioxidants to treat sepsis [115,116], which has higher bioavailability and efficacy in clinical practice. Such nanocarriers provide many benefits over conventional formulations, including superior solubility and stability, extended half-life, improved epithelial permeability and bioavailability, enhanced tissue targeting, and minimal side effects. Recently, Chen et al. developed a ROS-responsive polymeric nanocarrier of melatonin via the self-assembly of diblock copolymers of PEG and poly (propylene sulfide) (PPS) (Fig. 5D) [36]. PPS undergoes an oxidative conversion from hydrophobic to hydrophilic. As a result, the nanoparticles become swollen and disassemble when exposed to H2O2, and then release the encapsulated drugs. The melatonin nanocarriers significantly alleviate sepsis-induced liver injury by decreasing the hepatic lipid peroxidation level, tissue neutrophil accumulation, and inflammatory cytokine levels. The mechanism of melatonin activity was associated with decreased phosphorylation of the NF-κB p65 subunit, which could down-regulate the expression of inflammatory mediators, including IL-6, IL-1β, COX-2, iNOS, and NLRP3. In particular, the therapeutic effects of the melatonin nanocarriers were superior to free melatonin alone. Eventually, the liver function in septic mice improved and was accompanied by a decrease in the plasma levels of ALT and AST after treatment with the melatonin nanocarriers.
6. Inflammation regulation
Inflammation imbalance is the most critical mechanism responsible for the onset and progression of sepsis, which underlies the whole pathological process [94]. The inflammation response in sepsis can be divided into two different but overlapping stages. In the initial stage of sepsis, activated immune cells, such as macrophages and neutrophils, secrete a large amount of pro-inflammatory cytokines, causing excessive inflammation and damage by RONS [[117], [118], [119]]. In the later stages of sepsis, a prolonged or intense low-inflammatory state may lead to immune effector failure, eventually leading to immunosuppression. Therefore, inflammation regulation is necessary for the treatment of sepsis. Steroidal or non-steroidal anti-inflammatory drugs are often used for anti-inflammatory treatment, but these drugs are not effective for the treatment of sepsis. The latest anti-TNFα or anti-IL-1 monoclonal antibodies and other drugs have limited therapeutic effects on sepsis. The main reasons for the poor effect of these drugs in sepsis are non-specific distribution, short drug half-life, and low bioavailability. The development of well-designed nanomedicine creates some new prospects for the treatment of sepsis, as nanomaterials can be specially designed to adjust the expression of pro-inflammatory and anti-inflammatory molecules and can be given priority to enter target tissues from the site of administration, which can solve the disadvantages of off-target organ side effects and systemic toxicity. Different types of nanomaterials have been developed to control inflammation in sepsis, including nanocarrier devices for anti-inflammatory drugs, anti-inflammatory nanomaterials, and nanomaterials that actively target inflammatory cells (Fig. 6).
Fig. 6.
The balance between pro-inflammation cytokines (TNF-α, IL-3, IL-β, IL-6, IL-12, MIF and INF-γ) and anti-inflammation cytokines (IL-4, IL-10 and TGF-β) are significantly important in sepsis(A). The disordered homeostasis in sepsis can be regulated by nanomaterials through a variety of ways. It can be divided into three categories: (B) nanocarriers(chitosan derivatives, solid lipid nanoparticles, biomimetic nanoparticles) with anti-inflammation drugs can efficiently delivery anti-inflammation drugs (APS and Curcumin) into inflammation site; (C) nanoparticles with anti-inflammation capacity, including Au nanoparticles, SPIO nanoparticles, PLA/PLGA nanoparticles and macrophage/MSC nanovesicles; (D) nanomaterials targeting inflammatory cells (neutrophils and endothelial cells) could decrease the production of inflammation cytokines.
6.1. Nanocarriers with anti-inflammatory drugs
The therapeutic effects of traditional anti-inflammatory drugs are usually unsatisfactory in the practical applications [19,20]. The structure and physicochemical properties of anti-inflammatory drugs result in poor water solubility, low bioavailability, and difficulty with absorption. Drug delivery by nanoparticles has gradually become a suitable strategy that avoids the pharmacokinetic limitations of traditional drug formulations (Fig. 6B) [[120], [121], [122], [123]]. For example, astragalus polysaccharide (APS), an important natural anti-inflammatory drug, has been demonstrated to exert anti-inflammatory actions by regulating the TLR4-NF-κB signaling pathway. However, APS does not absorb well through the intestinal tract, and only a small amount is absorbed by the paracellular route. Xu et al. prepared APS nanoparticles using chitosan derivative (chitosan/tripolyphosphate) as the drug carrier [37]. The APS nanoparticle had better therapeutic effects than free APS alone, including a significant reduction in inflammatory cytokine levels and protection of the structure and function of cardiac tissue. Similarly, the clinical application of curcumin is limited due to its hydrophobic nature, rapid degradation, and low bioavailability. Wang et al. encapsulated curcumin in solid lipid nanoparticles, which not only protected the curcumin from chemical degradation, but also contributed to sustained drug release [39]. Consequently, curcumin-loaded nanoparticles exert a superior therapeutic effect compared to free curcumin, including inhibition of inflammatory cytokines, increased anti-inflammatory cytokines, and improved survival of septic mice.
Recent studies have also prepared biomimetic nanoparticles to further increase the bioavailability of anti-inflammatory drugs by coating the cell membrane on the surface of nanoparticles or incorporating proteins derived from the plasma membrane into nanoparticles [121,[40], [41], [124]]. Biomimetic nanoparticles can effectively avoid being cleared by the reticuloendothelial system and circulate in the body longer than ordinary nanomaterials [[40], [41], [124], [125]].
6.2. Anti-inflammatory nanoparticles
Some nanomaterials intrinsically exert anti-inflammatory activity, such as gold nanoparticles (AuNPs), superparamagnetic iron oxide nanoparticles (SPIONs), and poly (lactic acid) (PLA)/PLGA nanoparticles (Fig. 6C). Taratummarat et al. demonstrated that AuNPs can promote the polarization of M1 macrophages into M2 macrophages, induce the secretion of anti-inflammatory cytokines, and inhibit the expression of inflammatory cytokines [42]. Macrophages have been broadly classified into two groups: M1 macrophages that secrete pro-inflammatory cytokines, and M2 macrophages that secrete anti-inflammatory cytokines [126,127]. AuNPs improve the survival rate of septic mice by alleviating systemic inflammation [42]. Similarly, Xu et al. showed that SPIONs can enhance secretion of anti-inflammatory cytokine IL-10 by activating autophagy in macrophages, alleviating inflammation in septic mice [44]. Therefore, the application of these metal nanomaterials offers a new avenue for the treatment of sepsis. Recently, Casey et al. developed cargo-less PLA/PLGA nanoparticles to investigate their anti-inflammatory effects on septic mice [45]. The PLA/PLGA nanoparticles were hydrolyzed in the body to produce lactic acid, which could regulate the immune response [128,129]. The polymer nanoparticles not only significantly inhibited the secretion of inflammatory cytokines, but also influenced the maturation status of bone marrow-derived dendritic cells (BMDCs).
Some cells in the body, such as immune cells and stem cells, have anti-inflammatory effects. The biomimetic nanoparticles based on these cells also inherit the physiological properties of merocytes and perform specific functions, such as targeting inflamed tissues, and possess anti-inflammatory activity [124,130]. Macrophage-derived nanovesicles (NVs) and mesenchymal stromal cell (MSC)-derived NVs have been developed for the treatment of sepsis. Because they retain the anti-inflammatory phenotypes of macrophages and MSCs, they have been shown to significantly suppress the release of inflammatory cytokines and increase the levels of anti-inflammatory cytokines, ultimately improve sepsis-induced inflammatory damage [40,41].
6.3. Nanomaterials targeting inflammatory cells
The inflammatory response of sepsis is a very complex process that involves the innate immune system, the adaptive immune system, and endothelial system. In the innate immune system, phagocytosis-related cells, such as macrophages and neutrophils, play critical roles in removing pathogens [131]. However, inflammation can disrupt the neutrophil death program (apoptosis) to prolong neutrophils longevity, and the number of neutrophils rapidly increases in sepsis [117]. These neutrophils secrete massive amounts of inflammatory cytokines, which in turn cause neutrophil migration dysfunction. Subsequently, the deleterious accumulation of these dysfunctional neutrophils could cause tissue injury within remote vital organs [131,132]. Therefore, induction of neutrophil apoptosis is also an important aspect in alleviating sepsis-induced inflammatory damage (Fig. 6D). Recently, Zhang et al. synthesized pH-responsive bovine serum albumin (BSA) doxorubicin (DOX) nanoparticles (DOX-hyd-BSA NPs) via a pH-labile hydrazone bond. Neutrophils specifically bind to BSA because of the upregulated Fcγ receptors of neutrophils [47]. As a result, DOX-hyd-BSA NPs can effectively target neutrophils and deliver DOX into neutrophils. In addition, the acidic environment in neutrophils triggers the fracture of a pH-labile bond between albumin and DOX, releasing DOX from DOX-hyd-BSA NPs into neutrophils. Notably, DOX is a highly effective chemotherapeutic agent that can bind DNA bases to kill neutrophils [133]. The DOX-hyd-BSA NPs significantly inhibit the release of inflammatory cytokines from neutrophils and increase the survival of septic mice.
Endothelial cells play important roles in maintaining homeostasis in the body by coordinating anti-inflammatory, anti-coagulant, and anti-adhesive states [134]. However, excessive inflammation and RONS could damage endothelial cells, leading to their dysfunction and structural damage under septic conditions [74]. Therefore, inhibiting the inflammatory response of endothelial cells is also a crucial aspect of treating sepsis (Fig. 6D). Notably, recombinant activated protein C (APC), a component of the natural anticoagulant system, has been applied in the treatment of sepsis due to its potent anti-inflammatory activity for endothelial cells [135,136], but the severe bleeding tendency with APC due to the decomposition of procoagulant co-factors Va and VIIIa prevent its clinical application. Recently, Lee et al. designed a protein nanocage (TFMG) composed of short ferritin (sFn), γ-Carboxyglutamic Acid of Protein C (PC-Gla), PAR-1-activating peptide (TRAP), and a matrix metalloproteinase (MMP)-2 cleavage site, where sFn acts as a skeleton structure, inserting PC-Gla at the C-terminus and TRAP in the N-terminus [46]. The MMP-2 cleavage site was inserted between the sFn and PC-Gla to allow PC-Gla to escape from nanocomposites when they reach the MMP-activating sites. In particular, TRAP could activate PAR-1 to enhance Gla-ERPC-PAR-1-dependent signal transduction and switch from a pro-inflammatory to cytoprotective role in endothelial cells [135,137,138]. Because LPS can induce the activation and release of MMP-2 in endothelial cells, PC-Gla is released from nanocages at the site of inflamed endothelium after administration of TFMG. Subsequently, PC-Gla and TRAP bond to the ERPC and PAR-1 receptors, respectively, and then inhibit the release of inflammatory cytokines from leukocytes. Cell adhesion molecule (CAM) expression on the surface of endothelial cells promotes the adhesion and migration of leukocytes to inflamed endothelium. Notably, the TFMG protein nanocage significantly alleviates the vascular inflammatory response, with decreased vascular permeability and CAM expression, as well as inhibited migration of circulating leukocytes to the vascular endothelium. The survival rates in the TFMG-treated group were reported to be significantly higher than those in TFG (without the MMP-2 cleavage site) and free PC-Gla-TRAP groups.
7. Multifunctional nanomedicine
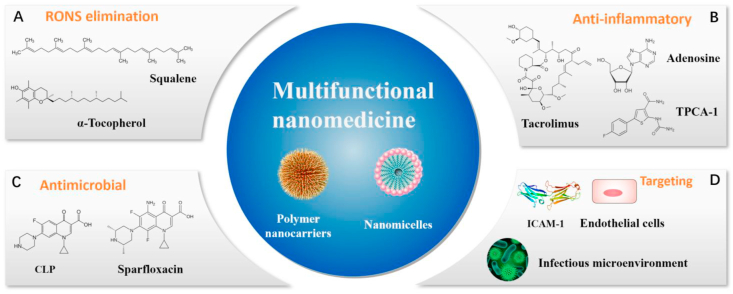
The course of sepsis is often the result of a combination of several factors that influence and interact with one another [10,139]. Therefore, therapies that only intervene with a single factor will compromise therapeutic effects and cannot effectively treat sepsis. Multifunctional nanomedicine can simultaneously deliver multiple therapeutic agents to interfere with multiple pathogenic factors. Thus, multifunctional nanomedicines to treat sepsis have great advantages and prospects for application (Fig. 7).
Fig. 7.
Multifunctional nanomedicine integrates multiple functions for treating sepsis, including (A) RONS elimination, (B) antibacterial, (C) anti-inflammatory, and (D)targeting.
The vicious circle between inflammation and RONS is a critical factor in the pathogenesis of sepsis [55,140]. Therefore, simultaneous intervention for inflammation and RONS can be an effective combination therapy. Recently, Dormont et al. prepared squalene (SQ)-based multidrug nanoparticles to simultaneously inhibit inflammation and scavenge RONS [48]. The nanoparticles were loaded with endogenous immunomodulator adenosine (Ad) and antioxidant α-tocopherol (vitamin E [VitE]) (SQAd/VitE NPs) (Fig. 7A and B). Ad, an endogenous purine, exerts anti-inflammatory effects by binding to the Ad receptor, but its efficacy is limited by the rapid clearance and severe side effects associated with off-target activation of the Ad receptor. VitE is an antioxidant that can eliminate excessive ROS in the body, but it is water-insoluble and generally binds to low-density lipoproteins (LDLs). The SQAd/VitE NPs not only improve the efficacy of these two drugs by prolonging circulation time, reducing toxicity, and improving targeting, but also exert the advantages of the combined drugs. Meanwhile, the interaction between neutrophils and the vascular endothelium results in an impaired endothelial barrier and increased permeability under excessive inflammation. Thus, SQAd/VitE NPs can selectively aggregate in areas of inflammation and endothelial injury. For the treatment of sepsis, SQAd/VitE NPs exert better therapeutic effects, including RONS scavenging and reducing secretion of pro-inflammatory cytokines (e.g., TNF-α and IL-6), in septic mice compared to exposure to free Ad/VitE, SQAd NPs or SQVitE NPs. Furthermore, SQAd/VitE NPs increase the generation of anti-inflammatory cytokine IL-10 and reduce malondialdehyde (MDA) levels, which is an indicator of lipid peroxidation in mice with sepsis. The SQAd/VitE NPs eventually improve the survival rate, increase systolic blood pressure, and decrease the murine sepsis score (MSS) in septic mice. Overall, SQAd/VitE NPs provide an effective treatment by blocking the pathological cross-talk between oxidative stress and inflammation.
Inflammation is the body's natural response to harmful stimuli [141], but excessive inflammation leads to dysfunction of the host's immune system [142,143]. Although anti-inflammatory drugs can reduce inflammation in patients with sepsis, they also lower the host's defenses and induce overactivation of pathogens and constant reproduction [20,139]. Thus, combined anti-inflammatory and antibacterial drugs can further enhance the treatment effect on sepsis. For example, Zhang et al. synthesized a pH/enzyme-responsive amphiphilic block copolymer consisting of biotinylated PEG-b-poly (β-amino ester)-b-PEG grafted with PEGylated lipid (Biotin-PEG-b-PAE(-g-PEG-b-DSPE)-b-PEG-Biotin) via Michael-type polymerization (Fig. 7 B-D) [49]. In particular, PAE (tertiary amines and ester bonds) and PEG-DSPE (phosphoester bonds) were pH-sensitive and enzyme-responsive in the amphiphilic block copolymer. The copolymer could self-assemble into micelles and encapsulate an antibiotic (ciprofloxacin [CLP]) and an anti-inflammatory agent (2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide [TPCA-1]). ICAM-1 antibodies bind to the surface of micelles. Therefore, the polymer nanoparticles (CIP + TPCA-1-NPs-anti-ICAM-1) could selectively accumulate in activated endothelial cells via the activity of the ICAM-1 antibody. Subsequently, the ester bonds of PAE and the phosphoester bonds of PEG-DSPE would break in the infectious microenvironment (low pH and abundant bacterial lipase enzymes). In the septic mouse model, CIP + TPCA-1-NPs-anti-ICAM-1 significantly suppresses peritoneal infection and protects vascular integrity. In addition, CIP + TPCA-1-NPs-anti-ICAM-1 effectively inhibits the inflammatory response of septic mice, reducing the leukocyte count and the levels of pro-inflammatory cytokines. Furthermore, CIP + TPCA-1-NPs-anti-ICAM-1 exhibits better therapeutic effects than free CIP, CIP-NPs-anti-ICAM-1, and CIP-NPs-anti-ICAM-1 by not only inhibiting bacterial proliferation and an inflammatory response, but also significantly increasing the survival rates of septic mice. Similarly, Yang et al. synthesized PLGA nanoparticles encapsulated with the antibiotic sparfloxacin (SFX) and anti-inflammatory immunosuppressant tacrolimus (TAC) [50]. The multidrug nanoparticles exhibited excellent bioavailability, low systemic toxicity, and an improved hemolysis ratio compared to free drugs. The γ3 peptide (NNQKIVNLKEKVAQLEA) attached to the surface of nanoparticles (SFX-TAC-γ3-PLGA nanoparticles) is a 17-mer linear peptide derived from the natural ligand of ICAM-1, which helps the nanoparticles selectively target inflamed endothelial cells. For the treatment of sepsis, SFX-TAC-γ3-PLGA nanoparticles selectively accumulate in the infectious and inflamed lung, and then inhibit bacterial proliferation and decrease the secretion of pro-inflammatory cytokines. Accordingly, SFX-TAC-γ3-PLGA nanoparticle treatment prevents cytokine storm and improves survival rates in septic mice.
8. The limitation of nanomaterials
The nanotherapies of sepsis are increased for the past few years, and present the exciting therapeutic effect and the great progresses. However, the issues of nanomaterials that involves toxicity and off-target was also brought forward and caught attentions.
Except the therapeutic effect, another crucial problem of nanotherapies of clinical translation is the toxicity. The toxicity of nanomaterials involves many aspects, which is closely related with their composition, size, surface charge and distribution in vivo. The nanomaterials for sepsis therapy dominantly divided to lipid-based nanomaterials, polymer nanomaterials and inorganic nanomaterials. Lipid-based nanomaterials generally have many advantages, like low toxicity, easy preparation, biocompatibility. Currently, lipid-based nanotherapies are the most commonly FDA-approved nanomedicines (such as for the therapy of ovarian cancer, fungal infection, and leukemia) [144,145]. Noteworthily, Sphingomyelin and cholesterol liposomal nanomaterials (Combioxin, SA) for toxin neutralization has been completed phase I clinical trials for the treatment of pneumonia sepsis [145]. Polymer nanomaterials generally have good biocompatible and can be biodegradable in vivo. Nanocarriers based polymers can achieve precise control of drug loading and release kinetics by regulation of their composition, stability, responsiveness, and surface charge. However, polymer nanoparticles easily cause increased aggregation in vivo, and have the risk of potential toxicity. Inorganic nanomaterials for sepsis treatment primary including cerium oxide, Au nanoparticle, iron oxide, 2D-TMD (WS2, MoS2), and silica. Particularly, iron oxide and MoS2 are all composed of elements already in the human body. MoS2 is already extensively applied to cancer diagnosis and therapy research [146]. Iron oxide has already authorized by FDA for nuclear magnetic contrast agent and the treatment of anemia [145]. These two materials are usually considered to have low toxicity. However, the toxicity of inorganic nanomaterials extremely caught attentions, especially the nanomaterials contained the elements of heavy mental. The distribution, metabolism, and toxicological action of the inorganic nanomaterials require in-depth study. In addition to the composition of nanoparticles, the size of nanoparticles also affects their toxicity. It has been reported that nanoparticles with small size are more likely to cause cytotoxicity. Nanoparticles with diameter less than approximately 10 nm can rapidly eliminated by the kidneys, which might affect the kidneys, and diameter greater than 200 nm may activate the complement system [147]. The surface of nanoparticles also influence toxicity. Cationic nanoparticles maybe caused cytotoxicity by damaged the cytomembrane. Specially, it has been reported the potential toxicity of nanomaterials about cardio- renal- and hepato-toxicity. For example, Abderrahim Nemmar et al. concluded that iron oxide nanoparticles can caused thrombosis, cardiac oxidative and DNA damage through acute intravenous administration [148]. There are also reports that titanium dioxide nanoparticles and others nanoparticles are harmful to kidney [149,150]. Tungsten trioxide nanoparticles and iron oxide nanoparticles can cause liver toxicity in mice [151,152]. There are many nanoparticles that can also damage the liver [153,154]. In general, the toxicity and therapeutic effects of nanomaterials are generally dose-dependent. Therefore, the dosage of nanomaterials needs to be carefully selected, and a balance should be reached between the toxicity of nanomaterials and the therapeutic effect. In addition, some researchers believe that concerns about the toxicity of nanomedicine have been exaggerated. It does not mean it is dangerous just because a material is nanoscale. In fact, nanoparticles were existed since the birth of the earth, for example, the nanoparticles were naturally existing in the organism, volcanic ash and ocean spray [155]. Moreover, human activity also produced excessive nanomaterials, such as dust and fume.
The current nanomaterials targeting for sepsis treatment mainly derived from the passive targeting (ERP) and specific targeting of modified ligands. The concept of ERP derived from tumors where the vasculature system is heterogeneous and abnormal in tumor site. ERP can cause the nanoparticles to accumulate in tumors [156]. Massive studies have reported that up to 10–15% of the injected NP accumulates at the tumor site compared with 0.1% of the free drug. However, Stefan Wilhelm et al. reported that only 0.7% of the nanoparticle injection dose reached the tumor [157]. Very recently, Lauren et al. argued that non-standard calculations was used by Stefan Wilhelm may produce misleading and biased results [158]. After the correction, the enrichment rate of nanoparticles in the tumor site ranged from 2.65 to 7.97%, much higher than the level of 0.7%. The oxidative stress and inflammatory cytokine storm of sepsis can severely damage the vascular endothelial system. Theoretically, nanoparticles should have better passive targeting in sepsis than in tumor. Nevertheless, future research must rigorously evaluate the metrics to quantify delivery and distribution. Currently, many nanotherapies are efficacious to treat sepsis in animal model, it may be not suitable for humans. There are great differences in the pathology and physiology of sepsis between humans and animals. These nanotherapies may be off-target in the treatment of human sepsis [159]. Lack of understanding of the differences between species may lead to the failure of nanotherapies in the treatment of sepsis in humans.
9. Summary and prospects
As the world continues to experience the effects of COVID-19, we present the latest advances in nanotherapy for sepsis with the aim of contributing to the treatment of this new, severe coronary disease. Here, we summarized recent advances in understanding the pathological mechanism of sepsis and the potential application of nanomedicines for sepsis treatment. Nanotherapies are promising for sepsis treatment, and the nanomaterials mediating RONS and the inflammatory response have achieved previously unattainable therapeutic effects in sepsis.
Despite the rapid advances, further optimization of nanomaterials is urgently needed to treat sepsis in a more comprehensive and effective manner. First, the pathogenesis of sepsis is the result of multiple factors, so single therapeutic agents are currently insufficient. However, the proposed multifunctional therapy is still in its infancy. Developing appropriate nanoparticles to meet the multifunctional requirements for sepsis treatment has become a crucial research focus. Second, ideal nanomaterials should not only have low toxicity and excellent bioavailability and stability, but they should also be able to easily target infectious tissues. The physical and chemical properties of nanomaterials are closely related to the particle size, surface charge, and surface modification [21]. Therefore, it is necessary to continuously optimize the preparation of nanoparticles. Third, inflammation in sepsis is caused primarily by the activation of TLRs and the NLR-mediated signaling pathway. However, recent studies on nanomaterials have focused mainly on blocking activation of the TLR signaling pathway. Yet, blocking the NLR-mediated signaling pathway can also inhibit activation of the innate immune system. Therefore, it is promising to design nanomaterials that block the NLR-mediated signaling pathway or both the TLR and NLR-mediated signaling pathways for the treatment of sepsis. Finally, many bacteria develop multidrug resistance and develop immune escape mechanisms in response to the host immune response and the abuse of antibiotic therapy [[160], [161], [162]]. Particularly in the immunosuppressive stage of late sepsis, there is a very complicated and contradictory relationship between regulating the level of inflammation and killing pathogens. Currently, most nanomaterials are focused on the first stage of sepsis (excessive activation of the inflammatory response) and are rarely involved in the late stage of sepsis. Nanomedicines should have great advantages and prospects through carefully designed multifunctional nanomaterials to balance the complex relationship between the late-stage regulation of inflammation and the elimination of pathogens. The development of new and effective nanomedicines for therapies based on inflammation and RONS will achieve a significant breakthrough in the treatment of sepsis [163,164]. The efficacy of nanomaterials therapy should be assessed in clinical trials.
Declaration of competing interest
The authors declare no conflict of interest, financial or otherwise.
Acknowledgements
This work was supported by the National Natural Science Foundation of China, China (No.21974134, 81974508, 81673492) and Innovation-Driven Project of Central South University (No. 202045005), Changsha Science and Technology Project (No. kq2001048), Key Research Project of Ningxia Hui Autonomous Region in 2021 (Major Project) (2021BEG01001).
Abbreviation
- ASC
Apoptosis associated speck-like containing a CARD domain
- ALT
Aminotransferase
- AST
Aspartate aminotransferase
- APS
Astragalus polysaccharide
- AuNPs
Gold nanoparticles
- APC
Activated protein C
- Ad
Adenosine
- BSA
Bovine serum albumin
- BMDCs
Bone-marrow-derived dendritic cells
- CAM
Cell adhesion molecule
- CLP
Ciprofloxacin/cecal ligation and puncture
- COX2
Cyclooxygenase-2
- CLRs
C-type lectin receptors
- COVID-19
Coronavirus disease 2019
- cfDNA
Cell free DNA
- DAMPs
Damage-associated molecular patterns
- DOX
Doxorubicin
- GPx
Glutathione peroxidase
- ICU
Intensive care units
- iNOS
Inducible nitric oxide synthase
- IL-1β
Interleukin-1β
- LDLs
Low-density lipoproteins
- LPS
Lipopolysaccharide
- MSC
Mesenchymal stromal cell
- MMP
Matrix metalloproteinase
- MDA
Malondialdehyde
- MSS
Murine sepsis score
- MSN
Mesoporous silica nanoparticles
- Mo
Molybdenum
- MODS
Multiple organ failure syndrome
- NOD
Nucleotide-binding oligomerization domain
- NLRs
NOD-like receptors
- NVs
Nanovesicles
- PAMPs
Pathogen-associated molecular patterns
- PC-Gla
γ-Carboxyglutamic Acid of Protein C
- PRRs
Pattern recognition receptors
- PLGA
Poly (lactic-co-glycolic acid)
- PEI
Polyethyleneimine
- PGE2
Prostaglandin E2
- PEG
Polyethylene glycol
- PCL
Poly(ε-caprolactone)
- PLA
Poly (lactic acid)
- PPS
Poly (propylene sulfide)
- RONS
Reactive oxygen and nitrogen species
- RLRs
Retinoic acid-inducible gene-like receptors
- ROS
Reactive oxygen species
- SIRS
Systemic inflammatory response syndrome
- SOD
Superoxide dismutase
- SAC
Single-atom catalysts
- sFn
short ferritin
- SQ
Squalene
- SFX
Sparfloxacin
- SPIONs
Superparamagnetic iron oxide nanoparticles
- TAC
Tacrolimus
- TNF-α
Tumor necrosis factor-α
- TMDs
Transition-metal dichalcogenides
- TD-NT
Telodendrimer nanotrap
- TLRs
Toll-like receptors
- VitE
Vitamin/α-tocopherol
- 6-AHA
6-aminohexanoic acid
- 2D
Two-dimensional
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work.
References
- 1.Li H. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395(10235):1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer M. The third international consensus definitions for Sepsis and Septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alhazzani W. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Crit. Care Med. 2020;48(6):e440–e469. doi: 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crunkhorn S. New route to sepsis therapy. Nat. Rev. Drug Discov. 2019;18(4) doi: 10.1038/d41573-019-00034-7. 251-251. [DOI] [PubMed] [Google Scholar]
- 5.Singer M. The third international Consensus definitions for sepsis and septic shock (Sepsis-3) J. Am. Med. Assoc. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao F. An enzyme-mimicking single-atom catalyst as an efficient multiple reactive oxygen and nitrogen species scavenger for sepsis management. Angew Chem. Int. Ed. Engl. 2020;59(13):5108–5115. doi: 10.1002/anie.201912182. [DOI] [PubMed] [Google Scholar]
- 7.Toledo A.G. Proteomic atlas of organ vasculopathies triggered by Staphylococcus aureus sepsis. Nat. Commun. 2019;10(1):4656. doi: 10.1038/s41467-019-12672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cecconi M. Sepsis and septic shock. Lancet. 2018;392(10141):75–87. doi: 10.1016/S0140-6736(18)30696-2. [DOI] [PubMed] [Google Scholar]
- 9.Venet F., Monneret G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat. Rev. Nephrol. 2018;14(2):121–137. doi: 10.1038/nrneph.2017.165. [DOI] [PubMed] [Google Scholar]
- 10.Gotts J.E., Matthay M.A. Sepsis: pathophysiology and clinical management. BMJ. 2016;353:i1585. doi: 10.1136/bmj.i1585. [DOI] [PubMed] [Google Scholar]
- 11.Prescott H.C., Angus D.C. Enhancing recovery from sepsis: a review. J. Am. Med. Assoc. 2018;319(1):62–75. doi: 10.1001/jama.2017.17687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vincent J.L., Mira J.P., Antonelli M. Sepsis: older and newer concepts. Lancet Respir Med. 2016;4(3):237–240. doi: 10.1016/S2213-2600(15)00522-6. [DOI] [PubMed] [Google Scholar]
- 13.Kim S.M. Fecal microbiota transplant rescues mice from human pathogen mediated sepsis by restoring systemic immunity. Nat. Commun. 2020;11(1):2354. doi: 10.1038/s41467-020-15545-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudd K.E. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleischmann C. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am. J. Respir. Crit. Care Med. 2016;193(3):259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 16.Hardeland R. Melatonin and inflammation-Story of a double-edged blade. J. Pineal Res. 2018;65(4) doi: 10.1111/jpi.12525. [DOI] [PubMed] [Google Scholar]
- 17.Fowler A.A., 3rd Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. J. Am. Med. Assoc. 2019;322(13):1261–1270. doi: 10.1001/jama.2019.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ritter C. Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit. Care Med. 2004;32(2):342–349. doi: 10.1097/01.CCM.0000109454.13145.CA. [DOI] [PubMed] [Google Scholar]
- 19.Hotchkiss R.S., Karl I.E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003;348(2):138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 20.Russell J.A. Management of sepsis. N. Engl. J. Med. 2006;355(16):1699–1713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 21.Jiang W. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008;3(3):145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y. Comprehensive insights into the multi-antioxidative mechanisms of melanin nanoparticles and their application to protect brain from injury in ischemic stroke. J. Am. Chem. Soc. 2017;139(2):856–862. doi: 10.1021/jacs.6b11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao Z.X. Antimicrobial gold nanoclusters eradicate Escherichia coli biofilms and are nontoxic by oral administration. Acs Appl. Bio Mater. 2020;3(8):5275–5286. doi: 10.1021/acsabm.0c00641. [DOI] [PubMed] [Google Scholar]
- 24.Liao F.H. Subnanometer gold clusters adhere to lipid A for protection against endotoxin-induced sepsis. Nano Lett. 2018;18(5):2864–2869. doi: 10.1021/acs.nanolett.7b05464. [DOI] [PubMed] [Google Scholar]
- 25.Thamphiwatana S. Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proc. Natl. Acad. Sci. U. S. A. 2017;114(43):11488–11493. doi: 10.1073/pnas.1714267114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chrishtop V.V. Organ-specific toxicity of magnetic iron oxide-based nanoparticles. Nanotoxicology. 2021;15(2):167–204. doi: 10.1080/17435390.2020.1842934. [DOI] [PubMed] [Google Scholar]
- 27.Shen S. Engineered nanoparticles disguised as macrophages for trapping lipopolysaccharide and preventing endotoxemia. Biomaterials. 2019;189:60–68. doi: 10.1016/j.biomaterials.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 28.Dawulieti J. Treatment of severe sepsis with nanoparticulate cell-free DNA scavengers. Sci. Adv. 2020;6(22) doi: 10.1126/sciadv.aay7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi C. A nanotrap improves survival in severe sepsis by attenuating hyperinflammation. Nat. Commun. 2020;11(1):3384. doi: 10.1038/s41467-020-17153-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selvaraj V. Inhibition of MAP kinase/NF-kB mediated signaling and attenuation of lipopolysaccharide induced severe sepsis by cerium oxide nanoparticles. Biomaterials. 2015;59:160–171. doi: 10.1016/j.biomaterials.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asano S. Cerium oxide nanoparticle treatment ameliorates peritonitis-induced diaphragm dysfunction. Int. J. Nanomed. 2015;10:6215–6225. doi: 10.2147/IJN.S89783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen G., Xu Y. Biosynthesis of cerium oxide nanoparticles and their effect on lipopolysaccharide (LPS) induced sepsis mortality and associated hepatic dysfunction in male Sprague Dawley rats. Mater. Sci. Eng. C Mater. Biol. Appl. 2018;83:148–153. doi: 10.1016/j.msec.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Soh M. Ceria-zirconia nanoparticles as an enhanced multi-antioxidant for sepsis treatment. Angew Chem. Int. Ed. Engl. 2017;56(38):11399–11403. doi: 10.1002/anie.201704904. [DOI] [PubMed] [Google Scholar]
- 34.Jeong H.G. Ceria nanoparticles fabricated with 6-aminohexanoic acid that overcome systemic inflammatory response syndrome. Adv. Healthc. Mater. 2019;8(9) doi: 10.1002/adhm.201801548. [DOI] [PubMed] [Google Scholar]
- 35.Yim D. Sustainable nanosheet antioxidants for sepsis therapy via scavenging intracellular reactive oxygen and nitrogen species. ACS Nano. 2020;14(8):10324–10336. doi: 10.1021/acsnano.0c03807. [DOI] [PubMed] [Google Scholar]
- 36.Chen G. Reactive oxygen species-responsive polymeric nanoparticles for alleviating sepsis-induced acute liver injury in mice. Biomaterials. 2017;144:30–41. doi: 10.1016/j.biomaterials.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Xu X. Protective effects of astragalus polysaccharide nanoparticles on septic cardiac dysfunction through inhibition of TLR4/NF-kappaB signaling pathway. Int. J. Biol. Macromol. 2020;153:977–985. doi: 10.1016/j.ijbiomac.2019.10.227. [DOI] [PubMed] [Google Scholar]
- 38.Naseri N., Valizadeh H., Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv. Pharmaceut. Bull. 2015;5(3):305–313. doi: 10.15171/apb.2015.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J. Anti-inflammatory activity of curcumin-loaded solid lipid nanoparticles in IL-1beta transgenic mice subjected to the lipopolysaccharide-induced sepsis. Biomaterials. 2015;53:475–483. doi: 10.1016/j.biomaterials.2015.02.116. [DOI] [PubMed] [Google Scholar]
- 40.Molinaro R. Macrophage-derived nanovesicles exert intrinsic anti-inflammatory properties and prolong survival in sepsis through a direct interaction with macrophages. Nanoscale. 2019;11(28):13576–13586. doi: 10.1039/c9nr04253a. [DOI] [PubMed] [Google Scholar]
- 41.Park K.S. Mesenchymal stromal cell-derived nanovesicles ameliorate bacterial outer membrane vesicle-induced sepsis via IL-10. Stem Cell Res. Ther. 2019;10(1):231. doi: 10.1186/s13287-019-1352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taratummarat S. Gold nanoparticles attenuates bacterial sepsis in cecal ligation and puncture mouse model through the induction of M2 macrophage polarization. BMC Microbiol. 2018;18(1):85. doi: 10.1186/s12866-018-1227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laurent S. Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: opportunities and challenges. Expet Opin. Drug Deliv. 2014;11(9):1449–1470. doi: 10.1517/17425247.2014.924501. [DOI] [PubMed] [Google Scholar]
- 44.Xu Y. SPIONs enhances IL-10-producing macrophages to relieve sepsis via Cav1-Notch1/HES1-mediated autophagy. Int. J. Nanomed. 2019;14:6779–6797. doi: 10.2147/IJN.S215055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Casey L.M. Cargo-less nanoparticles program innate immune cell responses to toll-like receptor activation. Biomaterials. 2019;218:119333. doi: 10.1016/j.biomaterials.2019.119333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee W. A double-chambered protein nanocage loaded with thrombin receptor agonist peptide (TRAP) and gamma-carboxyglutamic acid of protein C (PC-Gla) for sepsis treatment. Adv. Mater. 2015;27(42):6637–6643. doi: 10.1002/adma.201503093. [DOI] [PubMed] [Google Scholar]
- 47.Zhang C.Y. Nanoparticle-induced neutrophil apoptosis increases survival in sepsis and alleviates neurological damage in stroke. Sci. Adv. 2019;5(11) doi: 10.1126/sciadv.aax7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dormont F. Squalene-based multidrug nanoparticles for improved mitigation of uncontrolled inflammation in rodents. Sci. Adv. 2020;6(23):eaaz5466. doi: 10.1126/sciadv.aaz5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang C.Y., Gao J., Wang Z. Bioresponsive nanoparticles targeted to infectious microenvironments for sepsis management. Adv. Mater. 2018;30(43) doi: 10.1002/adma.201803618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y. Inflammation-targeting polymeric nanoparticles deliver sparfloxacin and tacrolimus for combating acute lung sepsis. J. Contr. Release. 2020;321:463–474. doi: 10.1016/j.jconrel.2020.02.030. [DOI] [PubMed] [Google Scholar]
- 51.van der Poll T. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 2017;17(7):407–420. doi: 10.1038/nri.2017.36. [DOI] [PubMed] [Google Scholar]
- 52.Jiang J.Y. Targeting NOX4 alleviates sepsis-induced acute lung injury via attenuation of redox-sensitive activation of CaMKII/ERK1/2/MLCK and endothelial cell barrier dysfunction. Redox Biol. 2020;36:14. doi: 10.1016/j.redox.2020.101638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu C.H., Liu H., Ge B. Innate immunity in tuberculosis: host defense vs pathogen evasion. Cell. Mol. Immunol. 2017;14(12):963–975. doi: 10.1038/cmi.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 55.Lugrin J. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014;395(2):203–230. doi: 10.1515/hsz-2013-0241. [DOI] [PubMed] [Google Scholar]
- 56.Rittirsch D., Flierl M.A., Ward P.A. Harmful molecular mechanisms in sepsis. Nat. Rev. Immunol. 2008;8(10):776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galley H.F. Oxidative stress and mitochondrial dysfunction in sepsis. Br. J. Anaesth. 2011;107(1):57–64. doi: 10.1093/bja/aer093. [DOI] [PubMed] [Google Scholar]
- 58.Breda C.N.S. Mitochondria as central hub of the immune system. Redox Biol. 2019;26:101255. doi: 10.1016/j.redox.2019.101255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bueno M. Mitochondria dysfunction and metabolic reprogramming as drivers of idiopathic pulmonary fibrosis. Redox Biol. 2020;33:101509. doi: 10.1016/j.redox.2020.101509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drose S., Brandt U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv. Exp. Med. Biol. 2012;748:145–169. doi: 10.1007/978-1-4614-3573-0_6. [DOI] [PubMed] [Google Scholar]
- 61.Di Florio D.N. Sex differences in inflammation, redox biology, mitochondria and autoimmunity. Redox Biol. 2020;31:101482. doi: 10.1016/j.redox.2020.101482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nolfi-Donegan D., Braganza A., Shiva S. Mitochondrial electron transport chain: oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020;37:101674. doi: 10.1016/j.redox.2020.101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang B., Chen Y., Shi J. Reactive oxygen species (ROS)-Based nanomedicine. Chem. Rev. 2019;119(8):4881–4985. doi: 10.1021/acs.chemrev.8b00626. [DOI] [PubMed] [Google Scholar]
- 64.Zhang L. Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 2019;26:101284. doi: 10.1016/j.redox.2019.101284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Forstermann U., Sessa W.C. Nitric oxide synthases: regulation and function. Eur. Heart J. 2012;33(7):829–837. doi: 10.1093/eurheartj/ehr304. 837a-837d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mitchell T., De Miguel C., Gohar E.Y. Sex differences in redox homeostasis in renal disease. Redox Biol. 2020;31:101489. doi: 10.1016/j.redox.2020.101489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leemans J.C. Pattern recognition receptors and the inflammasome in kidney disease. Nat. Rev. Nephrol. 2014;10(7):398–414. doi: 10.1038/nrneph.2014.91. [DOI] [PubMed] [Google Scholar]
- 68.Wen H., Miao E.A., Ting J.P. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity. 2013;39(3):432–441. doi: 10.1016/j.immuni.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song H. A high-loading drug delivery system based on magnetic nanomaterials modified by hyperbranched phenylboronic acid for tumor-targeting treatment with pH response. Colloids Surf. B Biointerfaces. 2019:182. doi: 10.1016/j.colsurfb.2019.110375. [DOI] [PubMed] [Google Scholar]
- 70.Li N. STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol. 2019;24:14. doi: 10.1016/j.redox.2019.101215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hotchkiss R.S., Monneret G., Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013;13(12):862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Incalza M.A. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018;100:1–19. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 73.Yang X., Chang Y., Wei W. Endothelial dysfunction and inflammation: immunity in rheumatoid arthritis. Mediat. Inflamm. 2016;2016:6813016. doi: 10.1155/2016/6813016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cross D. Epigenetics in sepsis: understanding its role in endothelial dysfunction, immunosuppression, and potential therapeutics. Front. Immunol. 2019;10:1363. doi: 10.3389/fimmu.2019.01363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin G.L. Epidemiology and immune pathogenesis of viral sepsis. Front. Immunol. 2018;9:2147. doi: 10.3389/fimmu.2018.02147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wiersinga W.J. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. J. Am. Med. Assoc. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 77.Yang L. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduct. Target Ther. 2020;5(1):128. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Y. Gram-scale synthesis of highly biocompatible and intravenous injectable hafnium oxide nanocrystal with enhanced radiotherapy efficacy for cancer theranostic. Biomaterials. 2020:226. doi: 10.1016/j.biomaterials.2019.119538. [DOI] [PubMed] [Google Scholar]
- 79.Cekerevac I. Predicting severity and intrahospital mortality in COVID-19: the place and role of oxidative stress. Oxid. Med. Cell Longev. 2021;2021:6615787. doi: 10.1155/2021/6615787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laforge M. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020;20(9):515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gu X. Respiratory viral sepsis: epidemiology, pathophysiology, diagnosis and treatment. Eur. Respir. Rev. 2020;29(157) doi: 10.1183/16000617.0038-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sweeney T.E., Wong H.R., Khatri P. Robust classification of bacterial and viral infections via integrated host gene expression diagnostics. Sci. Transl. Med. 2016;8(346):346ra91. doi: 10.1126/scitranslmed.aaf7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tang D. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol. Rev. 2012;249(1):158–175. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zindel J., Kubes P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu. Rev. Pathol. 2020;15:493–518. doi: 10.1146/annurev-pathmechdis-012419-032847. [DOI] [PubMed] [Google Scholar]
- 85.Jain V. Cationic nanoemulsions bearing ciprofloxacin surf-plexes enhances its therapeutic efficacy in conditions of E. coli induced peritonitis and sepsis. Pharm. Res. (N. Y.) 2014;31(10):2630–2642. doi: 10.1007/s11095-014-1360-0. [DOI] [PubMed] [Google Scholar]
- 86.Foit L., Thaxton C.S. Synthetic high-density lipoprotein-like nanoparticles potently inhibit cell signaling and production of inflammatory mediators induced by lipopolysaccharide binding Toll-like receptor 4. Biomaterials. 2016;100:67–75. doi: 10.1016/j.biomaterials.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 87.Fang R.H. Cell membrane coating nanotechnology. Adv. Mater. 2018;30(23) doi: 10.1002/adma.201706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li B. The potential of biomimetic nanoparticles for tumor-targeted drug delivery. Nanomedicine. 2018;13(16):2099–2118. doi: 10.2217/nnm-2018-0017. [DOI] [PubMed] [Google Scholar]
- 89.Luk B.T., Zhang L. Cell membrane-camouflaged nanoparticles for drug delivery. J. Contr. Release. 2015;220(Pt B):600–607. doi: 10.1016/j.jconrel.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Herrmann I.K. Endotoxin removal by magnetic separation-based blood purification. Adv. Healthc. Mater. 2013;2(6):829–835. doi: 10.1002/adhm.201200358. [DOI] [PubMed] [Google Scholar]
- 91.Wang L. A biocompatible method of decorporation: bisphosphonate-modified magnetite nanoparticles to remove uranyl ions from blood. J. Am. Chem. Soc. 2006;128(41):13358–13359. doi: 10.1021/ja0651355. [DOI] [PubMed] [Google Scholar]
- 92.Denning N.L. DAMPs and NETs in sepsis. Front. Immunol. 2019;10:2536. doi: 10.3389/fimmu.2019.02536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gong T. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020;20(2):95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 94.Huang M., Cai S., Su J. The pathogenesis of sepsis and potential therapeutic targets. Int. J. Mol. Sci. 2019;20(21) doi: 10.3390/ijms20215376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Banoth B., Cassel S.L. Mitochondria in innate immune signaling. Transl. Res. 2018;202:52–68. doi: 10.1016/j.trsl.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kang J.H. An extracorporeal blood-cleansing device for sepsis therapy. Nat. Med. 2014;20(10):1211–1216. doi: 10.1038/nm.3640. [DOI] [PubMed] [Google Scholar]
- 97.Ray P.D., Huang B.W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012;24(5):981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kang Q., Yang C. Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37:101799. doi: 10.1016/j.redox.2020.101799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Glass S.B. Redox potential and ROS-mediated nanomedicines for improving cancer therapy. Antioxidants Redox Signal. 2019;30(5):747–761. doi: 10.1089/ars.2017.7370. [DOI] [PubMed] [Google Scholar]
- 100.Lei X.G. Paradoxical roles of antioxidant enzymes: basic mechanisms and health implications. Physiol. Rev. 2016;96(1):307–364. doi: 10.1152/physrev.00010.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiang D. Nanozyme: new horizons for responsive biomedical applications. Chem. Soc. Rev. 2019;48(14):3683–3704. doi: 10.1039/c8cs00718g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang H., Wan K., Shi X. Recent advances in nanozyme research. Adv. Mater. 2019;31(45) doi: 10.1002/adma.201805368. [DOI] [PubMed] [Google Scholar]
- 103.Wu J. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II) Chem. Soc. Rev. 2019;48(4):1004–1076. doi: 10.1039/c8cs00457a. [DOI] [PubMed] [Google Scholar]
- 104.Huang Y., Ren J., Qu X. Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 2019;119(6):4357–4412. doi: 10.1021/acs.chemrev.8b00672. [DOI] [PubMed] [Google Scholar]
- 105.Corsi F. Not only redox: the multifaceted activity of cerium oxide nanoparticles in cancer prevention and therapy. Front Oncol. 2018;8:309. doi: 10.3389/fonc.2018.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Das S. Cerium oxide nanoparticles: applications and prospects in nanomedicine. Nanomedicine. 2013;8(9):1483–1508. doi: 10.2217/nnm.13.133. [DOI] [PubMed] [Google Scholar]
- 107.Pagliari F. Cerium oxide nanoparticles protect cardiac progenitor cells from oxidative stress. ACS Nano. 2012;6(5):3767–3775. doi: 10.1021/nn2048069. [DOI] [PubMed] [Google Scholar]
- 108.Amani H. Antioxidant nanomaterials in advanced diagnoses and treatments of ischemia reperfusion injuries. J. Mater. Chem. B. 2017;5(48):9452–9476. doi: 10.1039/c7tb01689a. [DOI] [PubMed] [Google Scholar]
- 109.Nelson B.C. Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants. 2016;5(2) doi: 10.3390/antiox5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Qiao B. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011;3(8):634–641. doi: 10.1038/nchem.1095. [DOI] [PubMed] [Google Scholar]
- 111.Xiang H., Feng W., Chen Y. Single-atom catalysts in catalytic biomedicine. Adv. Mater. 2020;32(8) doi: 10.1002/adma.201905994. [DOI] [PubMed] [Google Scholar]
- 112.Rajendrakumar S.K. Peroxidase-mimicking nanoassembly mitigates lipopolysaccharide-induced endotoxemia and cognitive damage in the brain by impeding inflammatory signaling in macrophages. Nano Lett. 2018;18(10):6417–6426. doi: 10.1021/acs.nanolett.8b02785. [DOI] [PubMed] [Google Scholar]
- 113.Hao J. In vivo long-term biodistribution, excretion, and toxicology of PEGylated transition-metal dichalcogenides MS2 (M = Mo, W, Ti) nanosheets. Adv. Sci. 2017;4(1):1600160. doi: 10.1002/advs.201600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pu J., Takenobu T. Monolayer transition metal dichalcogenides as light sources. Adv. Mater. 2018 doi: 10.1002/adma.201707627. [DOI] [PubMed] [Google Scholar]
- 115.Weissig V., Guzman-Villanueva D. Nanocarrier-based antioxidant therapy: promise or delusion? Expet Opin. Drug Deliv. 2015;12(11):1783–1790. doi: 10.1517/17425247.2015.1063611. [DOI] [PubMed] [Google Scholar]
- 116.Li Volti G. Antioxidant potential of different melatonin-loaded nanomedicines in an experimental model of sepsis. Exp. Biol. Med. 2012;237(6):670–677. doi: 10.1258/ebm.2012.011425. [DOI] [PubMed] [Google Scholar]
- 117.Shen X.F. Neutrophil dysregulation during sepsis: an overview and update. J. Cell Mol. Med. 2017;21(9):1687–1697. doi: 10.1111/jcmm.13112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gill R., Tsung A., Billiar T. Linking oxidative stress to inflammation: toll-like receptors. Free Radic. Biol. Med. 2010;48(9):1121–1132. doi: 10.1016/j.freeradbiomed.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chousterman B.G., Swirski F.K., Weber G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017;39(5):517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- 120.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015;33(9):941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sun D. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010;18(9):1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Novoselova E.G. Thymulin, free or bound to PBCA nanoparticles, protects mice against chronic septic inflammation. PloS One. 2018;13(5) doi: 10.1371/journal.pone.0197601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mishra P.R. An investigation on the approach to target lipopolysaccharide through polymeric capped nano-structured formulation for the management of sepsis. J. Biomed. Nanotechnol. 2011;7(1):47–49. doi: 10.1166/jbn.2011.1195. [DOI] [PubMed] [Google Scholar]
- 124.Molinaro R. Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat. Mater. 2016;15(9):1037–1046. doi: 10.1038/nmat4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu B. Precise engineering of neutrophil membrane coated with polymeric nanoparticles concurrently absorbing of proinflammatory cytokines and endotoxins for management of sepsis. Bioproc. Biosyst. Eng. 2020;43(11):2065–2074. doi: 10.1007/s00449-020-02395-5. [DOI] [PubMed] [Google Scholar]
- 126.Murray P.J. Macrophage polarization. Annu. Rev. Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 127.Shapouri-Moghaddam A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018;233(9):6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 128.Peter K. Lactic acid delays the inflammatory response of human monocytes. Biochem. Biophys. Res. Commun. 2015;457(3):412–418. doi: 10.1016/j.bbrc.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 129.Allen R.P. Latent, immunosuppressive nature of poly(lactic-co-glycolic acid) microparticles. ACS Biomater. Sci. Eng. 2018;4(3):900–918. doi: 10.1021/acsbiomaterials.7b00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Parodi A. Bio-inspired engineering of cell- and virus-like nanoparticles for drug delivery. Biomaterials. 2017;147:155–168. doi: 10.1016/j.biomaterials.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 131.Sonego F. Paradoxical roles of the neutrophil in sepsis: protective and deleterious. Front. Immunol. 2016;7:155. doi: 10.3389/fimmu.2016.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Drifte G. Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Crit. Care Med. 2013;41(3):820–832. doi: 10.1097/CCM.0b013e318274647d. [DOI] [PubMed] [Google Scholar]
- 133.Kaiser J. A colorful chemotherapy agent could be made less toxic. Science. 2020;369(6499):18. doi: 10.1126/science.369.6499.18. [DOI] [PubMed] [Google Scholar]
- 134.Li M. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 2018;831:52–59. doi: 10.1016/j.ejphar.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 135.Feistritzer C. Protective signaling by activated protein C is mechanistically linked to protein C activation on endothelial cells. J. Biol. Chem. 2006;281(29):20077–20084. doi: 10.1074/jbc.M600506200. [DOI] [PubMed] [Google Scholar]
- 136.Zhao X.Y. Targeted inhibition of activated protein C by a non-active-site inhibitory antibody to treat hemophilia. Nat. Commun. 2020;11(1):2992. doi: 10.1038/s41467-020-16720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Matthay M.A. Severe sepsis--a new treatment with both anticoagulant and antiinflammatory properties. N. Engl. J. Med. 2001;344(10):759–762. doi: 10.1056/NEJM200103083441009. [DOI] [PubMed] [Google Scholar]
- 138.Mosnier L.O., Zlokovic B.V., Griffin J.H. The cytoprotective protein C pathway. Blood. 2007;109(8):3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 139.Stearns-Kurosawa D.J. The pathogenesis of sepsis. Annu. Rev. Pathol. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Biswas S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell Longev. 2016;2016:5698931. doi: 10.1155/2016/5698931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Martinez J.O. Biomimetic nanoparticles with enhanced affinity towards activated endothelium as versatile tools for theranostic drug delivery. Theranostics. 2018;8(4):1131–1145. doi: 10.7150/thno.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hotchkiss R.S., Nicholson D.W. Apoptosis and caspases regulate death and inflammation in sepsis. Nat. Rev. Immunol. 2006;6(11):813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 143.Bosmann M., Ward P.A. The inflammatory response in sepsis. Trends Immunol. 2013;34(3):129–136. doi: 10.1016/j.it.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fenton O.S. Advances in biomaterials for drug delivery. Adv. Mater. 2018 doi: 10.1002/adma.201705328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Anselmo A.C., Mitragotri S. Nanoparticles in the clinic: an update. Bioeng. Transl. Med. 2019;4(3) doi: 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang J. MoS2-based nanocomposites for cancer diagnosis and therapy. Bioactive Mater. 2021;6(11):4209–4242. doi: 10.1016/j.bioactmat.2021.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hoshyar N. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine. 2016;11(6):673–692. doi: 10.2217/nnm.16.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nemmar A. Ultrasmall superparamagnetic iron oxide nanoparticles acutely promote thrombosis and cardiac oxidative stress and DNA damage in mice. Part. Fibre Toxicol. 2016;13(1):22. doi: 10.1186/s12989-016-0132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Brand W. Possible effects of titanium dioxide particles on human liver, intestinal tissue, spleen and kidney after oral exposure. Nanotoxicology. 2020;14(7):985–1007. doi: 10.1080/17435390.2020.1778809. [DOI] [PubMed] [Google Scholar]
- 150.Recordati C. Tissue distribution and acute toxicity of silver after single intravenous administration in mice: nano-specific and size-dependent effects. Part. Fibre Toxicol. 2016;13:12. doi: 10.1186/s12989-016-0124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mao L. A comparison of hepatotoxicity induced by different lengths of tungsten trioxide nanorods and the protective effects of melatonin in BALB/c mice. Environ. Sci. Pollut. Res. Int. 2021 doi: 10.1007/s11356-021-13558-6. [DOI] [PubMed] [Google Scholar]
- 152.Bellusci M. Biodistribution and acute toxicity of a nanofluid containing manganese iron oxide nanoparticles produced by a mechanochemical process. Int. J. Nanomed. 2014;9:1919–1929. doi: 10.2147/IJN.S56394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Khanna P. Nanotoxicity: an interplay of oxidative stress, inflammation and cell death. Nanomaterials. 2015;5(3):1163–1180. doi: 10.3390/nano5031163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sun T. Nanomaterials and hepatic disease: toxicokinetics, disease types, intrinsic mechanisms, liver susceptibility, and influencing factors. J. Nanobiotechnol. 2021;19(1):108. doi: 10.1186/s12951-021-00843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Griffin S. Natural nanoparticles: a particular matter inspired by nature. Antioxidants. 2018;7(1):3. doi: 10.3390/antiox7010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Scheetz L. Engineering patient-specific cancer immunotherapies. Nat. Biomed. Eng. 2019;3(10):768–782. doi: 10.1038/s41551-019-0436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wilhelm S. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016;1(5):16014. [Google Scholar]
- 158.Price L.S.L. A reanalysis of nanoparticle tumor delivery using classical pharmacokinetic metrics. Sci. Adv. 2020;6(29) doi: 10.1126/sciadv.aay9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pant A., Mackraj I., Govender T. Advances in sepsis diagnosis and management: a paradigm shift towards nanotechnology. J. Biomed. Sci. 2021;28(1):6. doi: 10.1186/s12929-020-00702-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cohen J. Sepsis: a roadmap for future research. Lancet Infect. Dis. 2015;15(5):581–614. doi: 10.1016/S1473-3099(15)70112-X. [DOI] [PubMed] [Google Scholar]
- 161.Morgan P., Majeed A. Sepsis: getting the balance right. BMJ. 2019;367:l6700. doi: 10.1136/bmj.l6700. [DOI] [PubMed] [Google Scholar]
- 162.Timsit J.F., Ruppe E., Ferrer R. Focus on sepsis: new concepts and findings in sepsis care. Intensive Care Med. 2018;44(11):1997–1999. doi: 10.1007/s00134-018-5406-3. [DOI] [PubMed] [Google Scholar]
- 163.Papafilippou L. Nanotools for sepsis diagnosis and treatment. Adv. Healthc. Mater. 2021;10(1) doi: 10.1002/adhm.202001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pant A., Mackraj I., Govender T. Advances in sepsis diagnosis and management: a paradigm shift towards nanotechnology. J. Biomed. Sci. 2021;28(1):6. doi: 10.1186/s12929-020-00702-6. [DOI] [PMC free article] [PubMed] [Google Scholar]