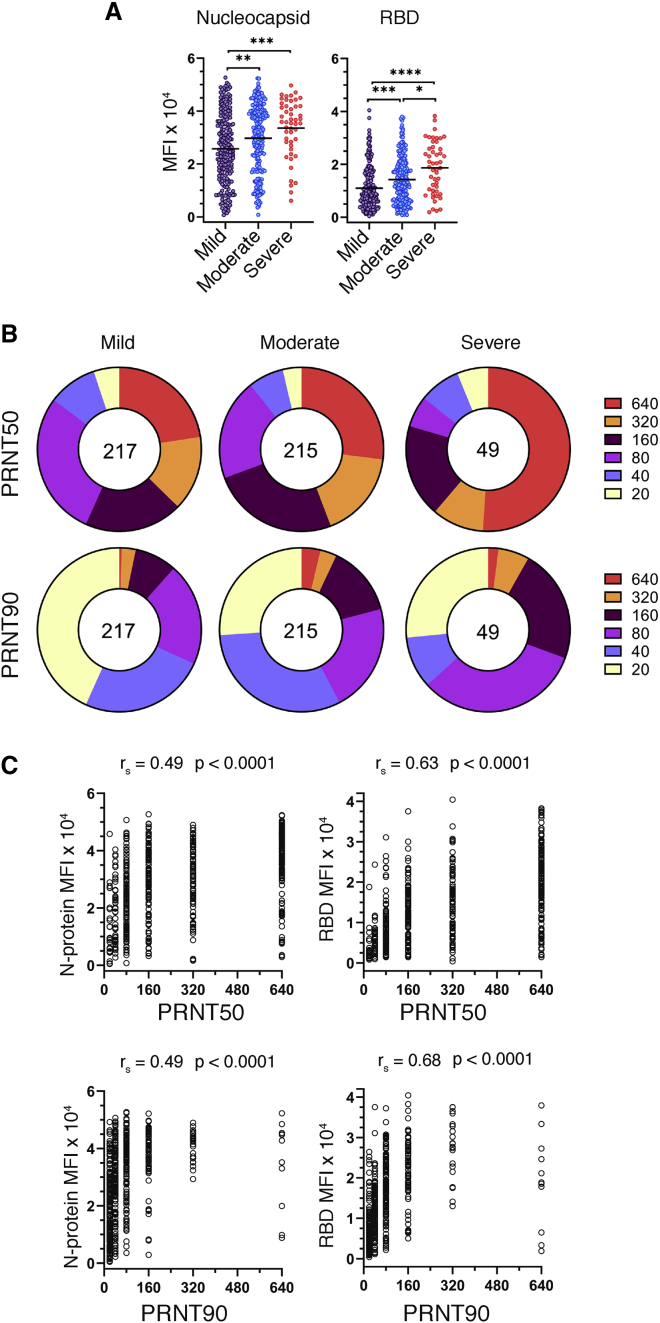
Figure 1.
Relationship of antibody production and virus neutralization capability with COVID-19 disease severity
Serum specimens from convalescent COVID-19 donors were analyzed for reactivity to SARS-CoV-2 antigens and neutralization capacity.
(A) Median fluorescence intensity (MFI) of total Ig (IgM, -A, and -G) reactivity to SARS-CoV-2 nucleocapsid and RBD as determined by a microsphere immunoassay on convalescent COVID-19 serum specimens, grouped by disease severity (n = 481). Statistical significance was determined by the non-parametric Kruskal-Wallis test, where ∗p < 0.05, ∗∗p < 0.001, ∗∗∗p < 0.0001, and ∗∗∗∗p < 0.00001 adjusted for multiple comparisons by Dunn’s test.
(B) Reciprocal plaque reduction neutralization titer (PRNT) 50 and 90 dilutions based on a live virus assay on convalescent COVID-19 individuals, grouped by disease severity (n = 481). The relative size of each pie slice represents the percentage of specimens with a given titer. The center number represents the number of specimens in each group.
(C) PRNT50 and PRNT90 titers plotted against SARS-CoV-2 nucleocapsid or RBD MFI. Graphs and Spearman’s correlations are based on the full cohort (n = 536) patient specimens.