Abstract
BACKGROUND
Essential hypertension is associated with increased plasma concentrations of extracellular vesicles (EVs). We aimed to determine the role of monocyte miR-27a in EVs on arterial Mas receptor expression, and its involvement in the pathogenesis of hypertension.
METHODS
THP-1 cells were transfected with miR-27a mimic and miR-27a inhibitor, and EVs were collected. Mas receptor expression and endothelial nitric oxide synthase (eNOS) phosphorylation were determined by immunoblotting. Sprague–Dawley (SD) rats received EVs via tail-vein injection. Blood pressure (BP) was measured with the tail-cuff method. The vasodilatory response of mesenteric arteries was measured using a small vessel myograph.
RESULTS
EVs from THP-1 cells increased rat BP by impairing Ang-(1–7)-mediated vasodilation in mesenteric arteries, which was further exaggerated by EVs from lipopolysaccharides-treated THP-1 cells. As the receptor and key signaling of Ang-(1–7), next experiments found that Mas receptor expression and eNOS phosphorylation were decreased in mesenteric arteries from EVs-treated SD rats. Screening studies found miR-27a in EVs may be involved in this process. Through transfection with miR-27a inhibitor or miR-27a mimic, we found that miR-27a downregulates Mas receptor expression in endothelial cells. Injection of EVs from miR-27a-transfected HEK-293 cells decreased Mas receptor and eNOS phosphorylation in mesenteric arteries, impaired Ang-(1–7)-mediated vasodilation and increased BP. Earlier effects were reversed using cells with downregulation of miR-27 in EVs.
CONCLUSIONS
Monocyte miR-27a in EVs decreases Mas receptor expression and eNOS phosphorylation in endothelium, impairs Ang-(1–7)-mediated vasodilation, and causes hypertension. Understanding the contributions of EVs in the pathogenesis of hypertension may facilitate their use as a diagnostic biomarker.
Keywords: blood pressure, eNOS, extracellular vesicles, hypertension, Mas receptor, miR-27a
Essential hypertension, one of the most common and important health problems worldwide, is a major risk factor for stroke, myocardial infarction, heart and kidney failure, and premature deaths.1 More recently, studies have paid more attention to the role of inflammation in the pathogenesis of hypertension.2 In fact, hypertension is now thought as a chronic low-grade inflammatory disease.3 Inhibition of inflammation may be a potential therapeutic strategy in hypertension. The classical renin–angiotensin system (RAS), which consists of a series of biological and enzymatic reactions resulting in the generation of angiotensin II (Ang II), is a vital system responsible for the regulation of blood pressure.4 Recent studies have focused on the new components of the RAS, such as Ang-(1–7). Ang-(1–7), via its G protein-coupled receptor Mas receptor, has several effects, including vasodilation and anti-inflammation.5,6 Many studies have shown that blood pressure in Mas gene-deleted mice is elevated,7–10 though studies also showed that administration of a Mas receptor antagonist A-779 does not affect the chronic hypertensive effects of Ang II in normal rats.11
Extracellular vesicles (EVs) are heterogeneous plasma membrane-derived vesicles released from most eukaryotic cells to the extracellular space in normal and disease states.12,13 On the basis of their size and secretory origin, EVs are categorized as exosomes, microvesicles, and apoptotic bodies. Exosomes are 30–100 nm in diameter and derived from the fusion of multivesicular bodies to the plasma membrane; microparticles, ranging from 100 to 1,000 nm, are thought to be the product of exocytic budding; apoptotic bodies are more than 1,000 nm in diameter and are formed when the cell undergoes programmed cell death.12,13 EVs are released into biological fluids, including interstitial, blood, and urine, and then transferred into the cytosol and nucleus in the recipient cells, even at a distance.14 Several pieces of evidence have shown that EVs are important in the pathogenesis of cardiovascular diseases, including atherosclerosis, heart failure, and hypertension.15–18
The association between EVs and hypertension is of current interest. In hypertensive patients, the plasma and urine EV levels are increased.18 However, it is still unclear whether or not EVs are involved in the pathogenesis of hypertension. The components of EVs are complex, including many functional molecules such as microRNAs (miRNA), messenger RNAs (mRNAs), proteins, DNA fragments, and lipids. On the basis of published data, comparison of plasma miRNA levels between hypertensive and healthy controls, and analysis of miRNA changes after lipopolysaccharides (LPS) treatment,19 we found that miR-27a is a candidate compound found in EVs, whose target gene is Mas receptor, predicted by TargetScan and miRTarBase softwares. Because of the important role of the Mas receptor in vascular endothelial function,20,21 we hypothesized that miR-27a in EVs is involved in hypertension by downregulation of Mas receptor expression and impairment of the vasodilatory effect of Ang-(1–7). Therefore, in this study, we sought to determine the role of monocyte miR-27a in EVs on arterial Mas receptor expression and function and its involvement in the pathogenesis of hypertension.
MATERIALS AND METHODS
Cell culture
Human acute monocytic leukemia (THP-1) cells [19] were purchased from cell bank of Chinese Academy of Sciences (Beijing, China). Human umbilical vein endothelial cells (HUVECs)22 and HEK-293 cells were purchased from American Type Culture Collection (ATCC, Gaithersburg, MD). All cells were cultured at 37 °C in 95% air and 5% CO2 atmosphere. HUVECs were cultured in Dulbecco’s modified eagle’s medium media supplemented with 1% penicillin/streptomycin/amphotericin-B and 10% fetal bovine serum (FBS; Fisher Scientific, Pittsburgh, PA). THP-1 cells were cultured in RPMI-1640 Media (HyClone, Logan, UT) and 10% FBS. To simulate the inflammatory state, THP-1 cells were treated with LPS (100 ng/ml) for 24 hours.23
EV isolation
EVs from cell culture supernatants were isolated and collected after a series of centrifugation, filtration, and ultracentrifugation steps according to our and other previous reports.24,25 Briefly, when the cells have grown to about 90% confluence, the cell culture supernatants were collected and centrifuged at 500 g for 20 minutes, and the initial pellets were discarded to remove residual cells. Then, the supernatants were centrifuged again at 1,500 g for 20 minutes, and the pellets were also discarded to remove any additional debris. After re-centrifugation at 110,000 g for 70 minutes, the final pellets containing EVs were resuspended in FBS-free medium. All steps were performed at 4 °C. The EVs were quantified by cell counting.
Electron microscopy
For negative staining transmission electron microscopy (TEM), the EVs were adsorbed to copper-coated mesh-grids for 2 minutes and rinsed in filtered phosphate-buffered saline (PBS). EVs on the grids were immediately fixed with 4% glutaraldehyde for 1 minute and then negatively stained with 2% (wt/vol) Na-phosphotungstate for 1 minute. Microscopic examinations were then carried out using Hitachi-7500 TEM (Hitachi, Soka City, Saitama, Japan), operated at 80 kV.24,26
Detection of EVs by flow cytometry
When THP-1 cells have grown to about 90% confluence, EVs and cells not pretreated with latex beads were counted using BD Accuri C6 flow cytometry (BD Biosciences, Franklin Lakes, NJ). RPMI 1640 cell culture medium was used as negative control. Data were analyzed using the BD Flow Plus software.27
RNA isolation and quantitative RT-PCR
Total RNA was extracted from cells or EVs using TRIzol reagent (Invitrogen, Carlsbad, CA). Quantitative real-time polymerase chain reaction (qRT-PCR) was then performed using TaqMan miRNA probes (Applied Biosystems, Foster City, CA), according to the manufacturer’s instructions. Briefly, total RNA was reverse-transcribed to complementary DNA, using reverse transcriptase and a stem-loop RT primer (TOYOBO, Tokyo Japan). RT-PCR was performed using a TOYOBO PCR kit and an 7300 Sequence Detection System (Applied Biosystems). All reactions were performed in triplicate, including no-template controls. After the reaction, the threshold cycle values were obtained using fixed threshold settings. A series of synthetic miRNAs, at known concentrations, were also reverse-transcribed and amplified to generate the standard curve. The expression levels of each target miRNA, obtained using the standard curve, were normalized by U6 snRNA.
Cell transfection
Synthetic pre-miR-27a, miR-27a mimic, miR-27a inhibitor, a mimic control, and short double-stranded RNAs were purchased from RiboBio (Guangzhou City, China). HEK-293 and THP-1 cells were transfected with equal amounts of synthetic miR-27a mimic, miR-27a inhibitor, and a mimic control RNA, using Lipofectamine 2000 (Invitrogen), according to the manufacturer’s instructions. The cells were harvested after 24 hours post-transfection.
Prediction of Mir-27a Targets
TargetScan (www.targetscan.com), starBase (http://starbase.sysu.edu.cn/), TarBase (http://microrna.gr/tarbase/), and miRBase (http://mirbase.org/index.shtml) were used to predict miR-27a targets. The targets were clustered by biological functions using blast2go (GO) tool (http://www.blast2go.com/b2ghome).
Luciferase assay
The full-length 3′-untranslated regions (UTR) of Mas receptor was cloned from human genomic DNA and inserted into psiCHECK-2 vector (Promega, Madison, WI) to generate psiCHECK-2–3′-UTR-Mas luciferase reporter system. Twenty-four hours before transfection, 1.2 × 104 cells were seeded into a 96-well plate. In brief, 10 pmol of miR-27a mimics or negative miRNA mimics (negative control) were co-transfected into cells with 100 ng of psiCHECK-2–3′-UTR-Mas, respectively, using DharmaFect Duo reagent (Dharmacon, Lafayette, CO). Luciferase assay was performed 24 hours after transfection, via the dual-luciferase reporter assay system (Promega, Madison, WI).28 Firefly luciferase activity was normalized to Renilla luciferase activity.
Immunoblotting
Cell lysates were boiled in sample buffer (35 mM Tris-HCl, pH 6.8, 4% SDS, 9.3% dithiothreitol, 0.01% bromophenol blue, and 30% glycerol) at 95 °C for 5 minutes. The proteins in samples, containing 50 μg cell protein, were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with 10% polyacrylamide gel, and then electroblotted onto nitrocellulose membranes (Bio-Rad, Berkeley, CA). The blots were blocked overnight with 5% nonfat dry milk in PBS-T (0.05% Tween 20 in 10 mM PBS) at 4 °C with constant shaking, and then incubated with antibodies in 5% nonfat dry milk in Tris-buffered saline/Tween buffer for 1 hour at room temperature. Those antibodies include Mas receptor (1:500; Santa Cruz Biotechnology, Santa Cruz, CA), eNOS, phospho-eNOS Ser1177, and phospho-eNOS Thr495 (1:1,000; Cell Signaling Technology, Danvers, MA). The membranes were washed 3 times with Tris-buffered saline/Tween buffer and then incubated with peroxidase-labeled goat anti-rabbit (1:15,000; Santa Cruz, CA) for 12 hour at 4 °C and developed for the detection of the specific protein using enhanced chemiluminescence reagents (Amersham, Little Chalfont, UK). The densities were normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:400; Santa Cruz, CA).
Animals
All Sprague–Dawley (SD) rats were housed in plastic cages and fed normal rodent chow and tap water at Daping Hospital. At 8 weeks of age, SD rats received tail-vein injections of PBS, normal THP-1 EVs (2 106), or LPS-treated THP-1 EVs (2 × 106) twice a week for 4 weeks. Blood pressure was measured weekly, using the tail-cuff method (ML125; PowerLab, AD Instruments, Castle Hill, Australia). All the rats were killed by an overdose of pentobarbital (100 mg/kg body weight) at the end of the experiment. The mesenteric arteries and plasma of the rats were collected for further experiments.
All study procedures were approved by the Third Military Medical University Animal Use and Care Committee. All experiments conformed to the guidelines of the ethical use of animals, and all efforts were made to minimize animal suffering and reduce the number of animals used.
Preparation and study of small resistance arteries
Male SD rats (230–240 g) were anesthetized with sodium pentobarbital (50 mg/kg), tracheotomized, and then the arterial blood samples were collected from the abdominal aorta. The entire mesenteric bed was removed carefully and placed in ice-cold physiological salt solution (PSS). The mesenteric arteries were dissected from the surrounding fat and connective tissues. Third-order branches of the superior mesenteric artery were cut into rings approximately 2 mm in length and mounted on 40 µm stainless-steel wires in Mulv any-Halpern small-vessel myograph (model M610, J.P. Trading, Science Park, Aarhus, Denmark).29,30 The rings were maintained in PSS at 37 °C and continuously bubbled with oxygen (95%) and carbon dioxide (5%), as in our previous reports. All dissecting procedures were carried out with extreme care to protect the endothelium from inadvertent damage. In one set of experiments, the endothelium was removed by pulling a hair along the vessel, and successful denudation of the endothelium was then confirmed by the absence of relaxation with the addition of acetylcholine (Ach, 10–6 M). At first, the rings were contracted with phenylephrine and high-potassium PSS (KPSS, 125 mM) to obtain the maximal response. When the response to phenylephrine reached a plateau, the response curves to Ang-(1–7) were measured by a cumulative concentration-dependent protocol (10–10 to 10–6 M). Responses to every single concentration of Ang-(1–7) were observed for 1 minutes. To test the specificity of the vasorelaxation caused by the Mas receptor, the rings were incubated with A779, a Mas receptor antagonist, for 30 minutes before the treatment with Ang-(1–7).31
Statistical analysis
The data are expressed as mean ± SEM. Comparison within groups was made by one-way analysis of variance (or paired t-test when only 2 groups were compared), and comparison among groups (or t-test when only 2 groups were compared) was made by factorial analysis of variance with Holm–Sidak test. A value of P < 0.05 was considered significant.
RESULTS
Extraction and identification of EVs
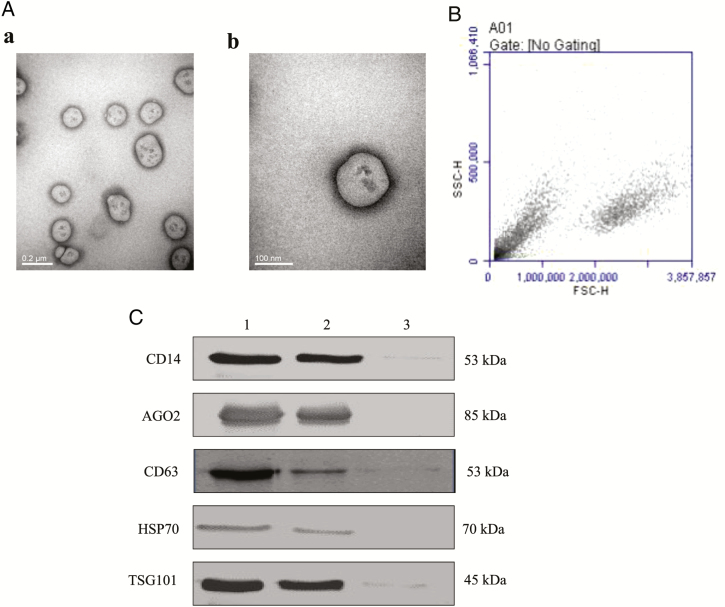
EVs from THP-1 cell culture supernatants were isolated and collected after a series of centrifugation, filtration, and ultracentrifugation24,25 and identified by several methods including TEM, flow cytometry analysis, and immunoblotting. First, the EVs in the pellets were visualized by TEM; they showed the characteristic phospholipid bilayer-enclosed EV structure, between 30 and 1,000 nm in diameter (Figure 1A), similar to previous reports.19,24 Then, EVs more than 200 nm were obtained by (Figure 1B). Finally, they were confirmed by immunoblotting for markers of EVs,32–34 such as CD14, argonaute 2 (AGO2), CD63, heat shock protein 70 (HSP70), and tumor susceptibility gene 101 (TSG101; Figure 1C).
Figure 1.
Identification and characterization of extracellular vesicles (EVs) from THP-1 cells. (A) Electron micrographs of EVs. EVs were isolated and collected after a series of centrifugation, filtration, and ultracentrifugation steps. EVs with different sizes from cell culture supernatants were observed under transmission electron microscopy (TEM). The EVs are 100–1,000 nm in diameter. The scale bars indicate 0.2 μm (a) and 100 nm (b). (B) flow cytometry (FCM) analysis. Cell samples and THP-1 EVs were analyzed by BD Accuri C6 FCM. Forward scatter (FSC), plotted by the cell size on the horizontal axis; side scatter (SSC), plotted by cellular components on the vertical axis. (C) CD14, AGO2, CD63, HSP70, and TSG101 expressions in EVs. EVs (1x107/mL, 200 µL), isolated from THP-1 cells, were separated on SDS-PAGE, electroblotted onto nitrocellulose membrane, and subjected to immunoblotting with antibodies against CD14 (1:500), AGO2 (1:2000), CD63 (1:500), HSP70 (1:400), and TSG101 (1:400). Lane 1: THP-1 cells; lane 2: EVs from THP-1 cells; and lane 3: RPMI 1640 cell culture medium as negative control.
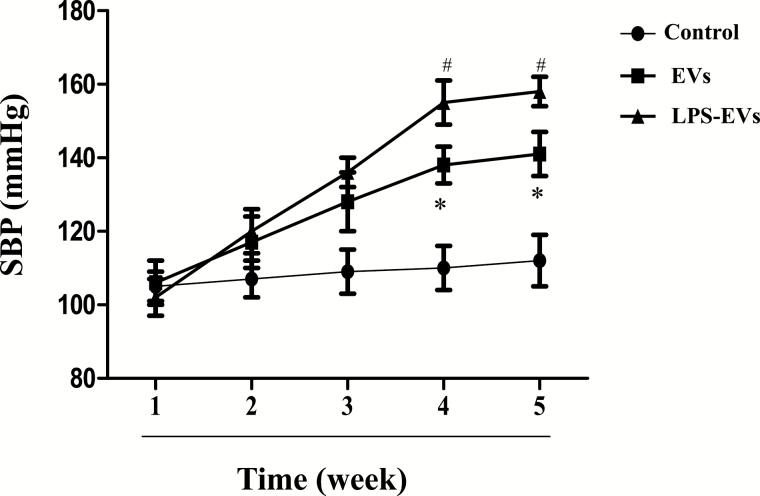
Increased blood pressure in SD rats treated with EVs from THP-1 cells
Previous studies have reported that EVs are increased in plasma or serum from hypertensive patients and rats with Ang II-induced hypertension.35–37 Therefore, EVs play an important role in the regulation of blood pressure. Our results showed that the systolic blood pressures of EV-treated SD rats were significantly higher than vehicle-treated rats; the significant difference was found after treatment for 4 weeks and remained elevated until the end of the study (Figure 2). To determine the consequence of inflammation on EV-mediated increase in blood pressure, we determined the effect of LPS-treated THP-1 cells on blood pressure. We found that treatment with LPS (100 ng/ml) for 24 hour increased the number of EVs in THP-1 cells (Supplementary Figure 1). Moreover, the systolic blood pressure was increased to a greater extent in SD rats injected with EVs from LPS-incubated THP-1 cells than those SD rats injected with EVs from vehicle-incubated THP-1 cells (Figure 2).
Figure 2.
The hypertensive effect of THP-1 extracellular vesicles (EVs) on Sprague–Dawley (SD) rats. After THP-1 cells were treated with lipopolysaccharides (LPS) (100 ng/ml) for 24 hour, EVs were collected. SD rats received tail-vein injections of phosphate-buffered saline (PBS), normal THP-1 EVs (2 × 106), or LPS-treated THP-1 EVs (2 × 106) twice a week for 5 weeks. Blood pressure was measured once per week using the tail-cuff method (ML125, PowerLab, AD Instruments, Castle Hill, Australia; n = 6/group, *P < 0.05 vs. control; #P < 0.05 vs. EVs).
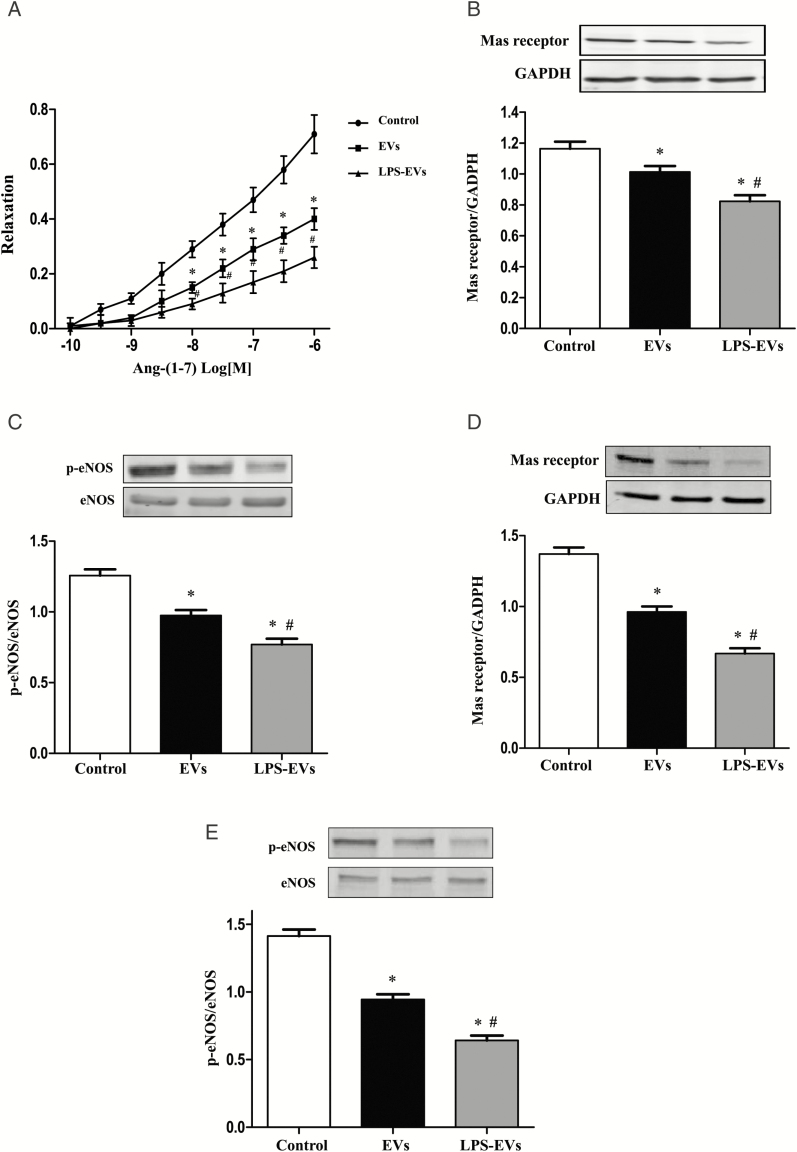
Impaired Mas receptor expression and function in the arteries of SD rats treated with EVs from THP-1 cells, which was worsened by LPS pretreatment
We next determined the effect of several vasoactive substances38,39 on vascular function in third-order branches of superior mesenteric arteries, such as Ang-(1–7), sodium nitroprusside, phenylephrine, and acetylcholine. We found that the vasoconstrictor effect of phenylephrine was increased in EV-treated rats; the vasoconstrictor effect was further increased in EVs treated with LPS (Supplementary Figure 2). Furthermore, we also found that Ang-(1–7)-induced (Figure 3A), but not sodium nitroprusside- and acetylcholine-induced (Supplemental Figures 3 and 4), vasodilation was impaired in phenylephrine-preconstricted mesenteric arteries from EVs-treated rats, which was worsened by EVs from LPS-incubated THP-1 cells (Figure 3A). The decreased vasodilatory effect of Ang-(1–7) was associated with decreased Mas receptor expression in the mesenteric arteries from EV-treated rats. The aggravation of the impaired vasodilatory effect of Ang-(1–7) with treatment of EVs from LPS-incubated THP-1 cells was associated with a further decrease in Mas receptor expression in the mesenteric arteries (Figure 3B). As a major pathway in Mas receptor signaling, the phosphorylation of eNOS was measured.40 We found that the phosphorylated eNOS expression of mesenteric arteries was decreased in EV-treated SD rats, which was decreased further after the injection of EVs from LPS-incubated THP-1 cells (Figure 3C).
Figure 3.
Impaired Mas receptor expression and function in the arteries of Sprague–Dawley (SD) rats treated with vehicle (control), or extracellular vesicles (EVs) from THP-1 cells treated with vehicle or LPS. (A) Effect of THP-1 EVs Ang-(1–7)-mediated vasodilation. After the SD rats were treated with EVs from THP-1 cells (2 × 106) or lipopolysaccharides (LPS)-treated THP-1 cells (2 × 106) twice a week for 4 weeks, third-order branches of mesenteric arteries were obtained. The mesenteric arterial rings were preconstricted with phenylephrine (10–5 M) and then treated with varying concentrations of Ang-(1–7) (10–10 to 10–6 M; n=6/group, *P < 0.05 vs. control; #P < 0.05 vs. EVs). (B and C) Effect of THP-1 EVs on the expression of Mas receptor and phosphorylated eNOS (p-eNOS) in the mesenteric artery of SD rats. SD rats received tail-vein injections of phosphate-buffered saline (PBS), normal THP-1 EVs (2 × 106), or LPS-treated THP-1 EVs (2 × 106) twice a week for 4 weeks. Third-order branches of mesenteric arteries of the rats were collected. Mas receptor (B) and p-eNOS (C) protein expressions were quantified by immunoblotting (n = 6/group, *P < 0.05 vs. control; #P < 0.05 vs. EVs). (D and E) Effect of THP-1 EVs on the expression of Mas receptor and p-eNOS in recipient human umbilical vein endothelial cells (HUVECs). HUVECs were incubated with vehicle (control), normal THP-1 EVs (2 × 106) or LPS-treated THP-1 EVs (2 × 106) for 24 hour. Mas receptor (D) and p-eNOS (E) protein expressions were quantified by immunoblotting (n = 6/group, *P < 0.05 vs. control; # P < 0.05 vs. EVs).
The results of the in vivo experiments were corroborated by in vitro experiments. Compared with vehicle treatment, treatment with EVs (2 × 106, 24 hours) decreased Mas receptor expression and eNOS phosphorylation in cultured HUVECs. These effects were worsened after incubation with EVs from LPS (100 ng/ml, 24 hour) treated THP-1 cells (Figures 3D and 3E).
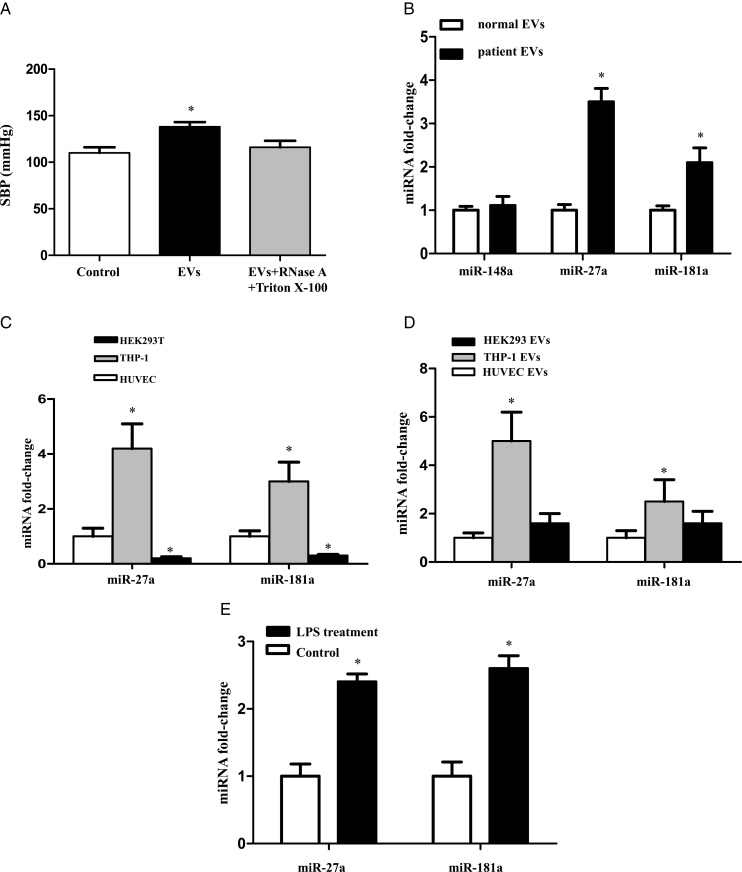
MiR-27a in EVs is involved in the elevation of blood pressure caused by injection of EVs
EVs contain components of cells, including extracellular receptors and ligands, lipids on the outside and cytoplasmic proteins and RNAs (mRNA, miRNA, and others) inside.12–14 To determine which component(s) play an important role in the regulation of blood pressure in vivo, EVs were treated with Triton X-100 (0.5%) and incubated with RNase (100 µg/ml) for 30 minutes. The RNase-treated and untreated EVs were injected via the tail vein twice a week for 4 weeks in SD rats. Injection of RNase-treated EVs prevented the increase in systolic blood pressures caused by EV treatment of SD rats (Figure 4A). Thus, RNAs, including miRNAs, may play a vital role in the EV-induced elevation of blood pressure.
Figure 4.
Micro RNAs (miRNAs) involved in the extracellular vesicle (EV)-mediated regulation of blood pressure. (A) Role of RNA in the EV-mediated regulation of blood pressure. EVs from THP-1 cells were treated with or without Triton X-100 (0.5%) and RNase (100 μg/ml) for 30 minutes. The RNase-treated and untreated EVs were injected via the tail vein twice a week for 4 weeks in Sprague–Dawley (SD) rats. Systolic blood pressure of SD rats was measured by the tail-cuff method (n = 6/group, * P< 0.05 vs. others). (B) The expressions of miR-148a, miR-27a, and miR-181a in EVs of healthy subjects and hypertensive patients. After the plasma (30 ml) of healthy subjects and hypertensive patients was collected, EVs were isolated. The expressions of miR-148a, miR-27a, and miR-181a were determined by qRT-PCR (n = 6/group,*P < 0.05 vs. EVs from healthy subjects). (C) The expressions of miR-27a and miR-181a in EVs from different cells. EVs were collected from human umbilical vein endothelial cells (HUVECs), THP-1 cells, and HEK-293 cells that were cultured for 24 hour. The expressions of miR-27a and miR-181a were quantified by qRT-PCR. The corresponding miRNA expressions in HUVECs were used as a control (n = 6/group, *P <0.05 vs. others; #P < 0.05 vs. EVs from HUVECs). (D) The expressions of miR-27a and miR-181a in HUVECs treated with EVs from different cells. HUVECs were incubated with EVs from HEK-293 cells or THP-1 cells for 24 hour. The expressions of miR-27a and miR-181a were quantified by qRT-PCR. The miRNA expressions in untreated HUVECs were used as a control (n = 6/group,*P < 0.05 vs. others). (E) Comparison of miR-27a and miR-181a expressions in EVs from vehicle (control) and human umbilical vein endothelial cells (LPS)-treated THP-1 cells. EVs were isolated from THP-1 cells treated vehicle or LPS (100 ng/ml) for 24 hour. The expressions of miR-27a and miR-181a were quantified by qRT-PCR (n = 6, *P < 0.05 vs. control).
EVs from THP-1 cells contain a variety of miRNAs,30 including miR-148a, miR-150, miR-181a, miR-124a, and miR-27a. Among these miRNAs, 3 miRNAs, miR-148a, miR-181a, and miR-27a, are associated with the RAS.41–43 To distinguish which miRNAs are involved in the EVs-induced increased blood pressure, we first compared the expressions of those 3 miRNAs in plasma from hypertensive patients and healthy subjects (Supplementary Table 1). Both miR-27a and miR-181a expressions were higher in hypertensive patients than healthy subjects, whereas there was no difference in miR-148a levels between the 2 groups (Figure 4B). Therefore, miR-148a was excluded from the candidate list.
We, next, investigated the endogenous expressions of miRNAs in HEK-293 and THP-1 cells, and HUVECs. Among the 3 cell lines, the levels of miR-181a and miR-27a were highest in THP-1 cells (Figure 4C). HUVECs were then incubated with EVs from HUVECs, THP-1 cells, and HEK-293 cells. Only EVs from THP-1 cells, not HUVECs and HEK-293 cells, increased the miR-27a and miR-181a levels in the recipient HUVECs (Figure 4D). We also found that incubation of THP-1 cells with LPS (100 ng/ml, 24 hour) further increased the expressions of both miR-27a and miR-181a (Figure 4E), suggesting the possibility that miR-27a and miR-181a are involved in the EV-mediated regulation of blood pressure.
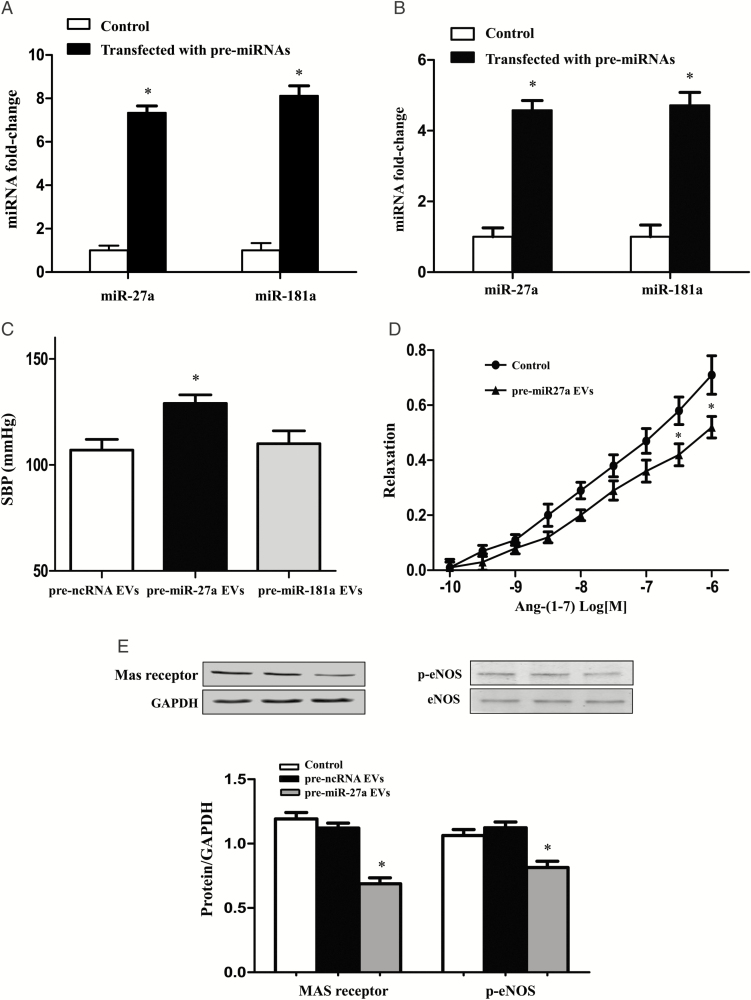
To determine the role of miR-27a and miR-181a in the regulation of blood pressure, HEK-293 cells were transfected with pre-miR-27a or pre-miR-181a. The increased expressions of the 2 miRNAs were confirmed in whole cells (Figure 5A) and their corresponding EVs (Figure 5B) by RT-PCR. Then, the EVs were injected into SD rats via the tail vein twice a week for 4 weeks. The results showed that pre-miR-27a EVs, not pre-miR-181a EVs, increased the systolic blood pressure of SD rats (Figure 5C). Further studies found that in pre-miR-27a EV-treated-SD rats, Ang-(1–7)-induced vasodilation was impaired in the mesenteric arteries preconstricted with phenylephrine (Figure 5D). Consistent with the results of EVs from THP-1 cells (Figures 3B-3E), pre-miR-27a EV also decreased the expressions of the Mas receptor and p-eNOS in mesenteric arteries (Figures 5E).
Figure 5.
Effect of miR-27a in extracellular vesicle (EV)-mediated increase in blood pressure. (A and B) The expressions of miR-27a and miR-181a in HEK-293 cells or EVs from HEK-293 cells transfected with pre-miR-27a or pre-miR-181a for 24 hour. The expressions of miR-27a and miR-181a in HEK-293 cells (A) or EVs from HEK-293 cells (B) were determined by qRT-PCR. The miRNA expressions in untransfected cells were used as a control (n = 3/group, *P < 0.05 vs. control). (C) Effect of miR-27a and miR-181a on the blood pressure. EVs were collected after HEK-293 cells were transfected with pre-ncRNA (control), pre-miR-27a, and pre-miR-181a for 24 hours. Then EVs were injected into Sprague–Dawley (SD) rats via the tail vein, twice a week for 4 weeks. Blood pressure was measured once a week by the tail-cuff method (n = 3/group, *P < 0.05 vs. others). (D) Effect of miR-27a on the regulation of Ang-(1–7)-mediated vasodilation. After SD rats were treated with EVs (2 × 106) from HEK-293 cells transfected with pre-miR-27a or ncRNA twice a week for 4 weeks, third-order branches of mesenteric arteries were obtained. Mesenteric arterial rings were preconstricted with phenylephrine (10–5 M) and then treated with varying concentrations of Ang-(1–7) (10–10 to 10–6 M; n = 3/group,*P < 0.05 vs. control). (E) Effect of EVs from HEK-293 transfected with pre-miR-27a on the expressions of Mas receptor and p-eNOS in the mesenteric arteries of SD rats. After SD rats were treated with EVs (2 × 106) from HEK-293 transfected with vehicle (control), pre-miR-27a or ncRNA twice a week for 4 weeks, mesenteric arteries were isolated. The protein expressions of Mas receptor and p-eNOS were quantified by immunoblotting (n = 3, *P < 0.05 vs. control).
Mas receptor is the target protein of monocyte miR-27a in EVs
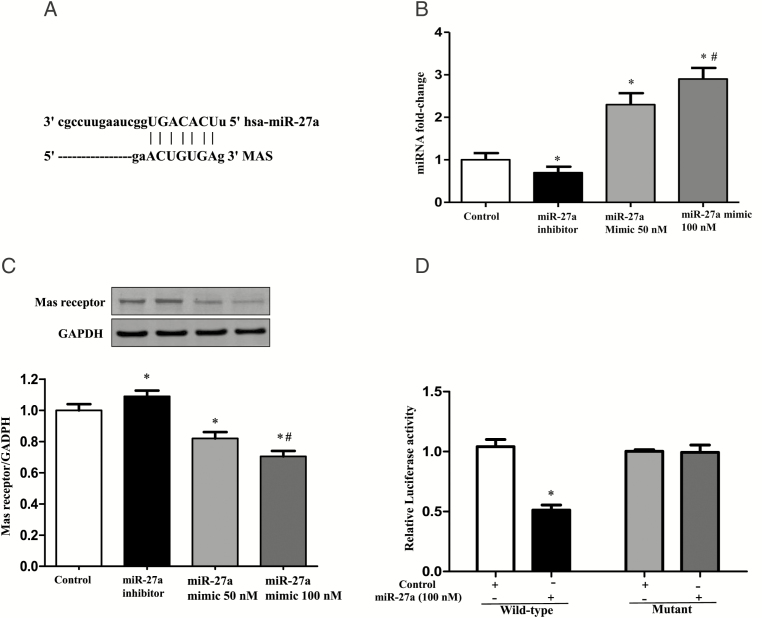
Bioinformatics data confirmed that Mas receptor is one of the targets of miR-27a, predicted by TargetScan and miRTarBase softwares, supporting the hypothesis that miR-27a is a candidate for EV-induced hypertension. Genomic alignment showed that there is a miR-27a binding site in the 3′-UTR of the Mas receptor (Figure 6A). We, then, investigated the regulation of Mas receptor expression by miR-27a in vitro. THP-1 cells were transfected with a miR-27a inhibitor or a miR-27a mimic; the successful cell transfection was determined in the EVs from individual cells (Figure 6B). EVs from above transfected THP-1 cells were incubated with the HUVEC cells, and then expression of Mas receptor was examined. Results showed that the miR-27a inhibitor increased Mas receptor expression, whereas the overexpression of miR-27a decreased Mas receptor expression in the recipient HUVECs (Figure 6C), indicating that miR-27a negatively regulates the expression of Mas receptors.
Figure 6.
Effect of monocyte miR-27a on the regulation of Mas receptor expression. (A) Genomic alignment showed of miR-27a binding site in the 3′-UTR of the Mas receptor predicted by TargetScan and miRTarBase softwares. (B) The expression of miR-27a in extracellular vesicles (EVs) from THP-1 cells treated with miR-27a inhibitor or mimic. EVs were collected from THP-1 cells transfected with miR-27a mimic (50 nM and 100 nM) or miR-27a inhibitor (100 nM) for 24 hours. The expression of miR-27a in EVs was quantified by qRT-PCR (n = 3/group, *P < 0.05 vs. Control, # P < 0.05 vs. 50 nM miR-27a mimic). (C) The protein expression of Mas receptor in HUVEC cells incubated with EVs from THP-1 cells transfected with miR-27a mimic and miR-27a inhibitor. THP-1 cells were transfected with miR-27a mimic (50 nM and 100 nM) and miR-27a inhibitor (100 nM) for 24 hours, and then EVs were collected, which were next incubated with the human umbilical vein endothelial cells (HUVECs) for 24 hours. Mas receptor protein expression was quantified by immunoblotting (n = 3/group, *P < 0.05 vs. Control, #P < 0.05 vs. 50 nM miR-27a mimic). (D) Luciferase analysis of the effect of miR-27a mimic on the regulation of Mas receptor expression. Luciferase activity was measured in HEK-293 cells co-transfected with miR-27a, pMIR-MAS-3′-UTR (wild-type), or pMIR-MAS-3′-UTR (mutant) treated with vehicle (control) or miR-27 mimic (100 nM; n = 6/group, *P < 0.05 vs. control).
To test whether or not the regulation of miR-27a on Mas receptor is via a direct or indirect mechanism, we constructed luciferase reporters with entire wild-type Mas 3′-UTR or a mutant Mas 3′-UTR. Following their confirmation by sequencing, they were co-transfected into HEK-293 cells with miR-27a mimic (100 nM) or mimic (Vehicle) control. We found that neither the mimic controls nor the mutant miR-27 3′-UTR had any effect on the luciferase activity in transfected HEK-293 cells. By contrast, miR-27a mimic decreased the luciferase activity in HEK-293 cells transfected with Mas–3′-UTR-wild-type (Figure 6D).
DISCUSSION
The effect of EVs on the regulation of blood pressure is still unclear. In our present study, we found that the blood pressure of SD rats was higher after intravenous injection of EVs from THP-1 cells. The EV-mediated increase in blood pressure in the SD rats was enhanced after the intravenous injection of EVs from THP-1 cells incubated with LPS treatment, presumably related to inflammation.
To uncover the mechanisms underlying the role of EVs in the regulation of blood pressure, we studied the effect of several vasodilators and phenylephrine on the mesenteric artery, and found that Ang-(1–7)-induced but not nitroprusside- and acetylcholine-induced vasodilation was impaired in phenylephrine-preconstricted mesenteric arteries from EVs-treated rats, which was aggravated by using EVs from LPS-incubated THP-1 cells. Ang-(1–7) is a product of the cleavage of Ang-II by angiotensin-converting enzyme 2, prolylcarboxypeptidase, and prolyl endopeptidase.44 By contrast to Ang II, Ang-(1–7), via its Mas receptor, exhibits vasodilatory, natriuretic, and diuretic effects, leading to a lowering blood pressure.44,45 We found the expressions of both Mas receptor and the phosphorylation of eNOS, one of its signaling pathway, were decreased in the mesenteric arteries in EV-treated SD rats, which was worsened after treatment with EVs from LPS-incubated THP-1 cells. These suggested that monocyte EVs, by impairing Mas receptor expression and function, are involved in the pathogenesis of hypertension.
As mentioned earlier, EVs contain proteins, lipids, genetic material such as genomic and mitochondrial DNAs, small and long-coding and non-coding RNA (mRNA, miRNA, and lncRNA), and other cytosolic components and molecules.12–17 After degradation of EV RNAs with Triton X-100 and RNAase, the increased blood pressures in the EVs-treated SD rats were attenuated, suggesting the role of RNAs, possibly miRNAs, in the EVs-induced hypertension. Several studies have shown a relationship between miRNAs and hypertension.46,47 Moreover, the expression of certain urinary exosomal miRNAs is associated with the blood pressure response to sodium intake.48 Vascular miRNAs also mediate mineralocorticoid receptor-induced vasoconstriction and the increase in blood pressure with aging.49 In this study, we showed that miR-27a EVs, not miR-181a or miR-148a, from THP-1 cells increased the systolic blood pressure of SD rats, indicating a role of miR-27a in mediating the increase in blood pressure caused by monocyte EVs.
It is well known that RAS plays a vital role in the regulation of blood pressure.4–6 There are some reports showing that miRNAs are involved in the regulation of RAS.41–43 For example, miRNAs can regulate the expression of AT1R and endothelial angiotensin-converting enzyme and modulate Ang II-induced endothelial inflammation, migration and ERK1/2 activation.50–52 Studies have shown abundant levels of mir-27a in blood and plasma exosomes, which are increased in individuals with the metabolic syndrome.53 MiR-27a is also reported to be associated with the regulation of RAS.43,54,55 Our results showed increased miR-27a level in THP-1 cells, which was further enhanced after treatment of LPS. Importantly, bioinformatics data predicted the Mas receptor as a target of miR-27a; there is a miR-27a binding site in 3′-UTR of the Mas receptor. In loss- and gain-of-function experiments, we found that a miR-27a inhibitor increased, whereas miR-27a overexpression decreased Mas expression in HUVECs. Our results also showed that a miR-27a mimic inhibited relative luciferase activity of Mas receptor 3′-UTR vector containing wild-type, but not mutant binding site of miR-27a, which suggests that Mas receptor is a direct target of miR-27a. The alteration of miR-27a expression in EVs has physiological significance because injection of EVs from miR-27a-transfected HEK-293 cells increased the blood pressure in SD rats. Moreover, Ang-(1–7) induced-vasodilation was impaired by miR27a in mesenteric arteries preconstricted by phenylephrine. These results show that miR-27a from monocyte EVs negatively regulate Mas receptor expression and function, i.e., vasodilation.
There are 3 limitations to our study. First, our present study could not distinguish the contribution of EV subtypes, including exosome, microvesicle, or apoptotic body. Second, Mas receptor knockout mice should be used in the future to demonstrate the importance of Mas receptor in the elevated blood pressure. Third, we only measured eNOS phosphorylation, direct measurement of nitric oxide production should be performed in the future.
In summary, our study shows direct evidence supporting the hypothesis that monocyte miR-27a in EVs decreases Mas receptor expression, phosphorylation of eNOS, and impairs Ang-(1–7)-mediated vasodilation, which may be involved in the pathogenesis of hypertension. Therefore, in clinic, we not only pay attention to the monocyte itself but also need to realize the importance of EV and its contents in the development of hypertension, which might be resistant to the medicine. Finding a therapeutic strategy to attenuate the effect of those factors in EVs, which elevate blood pressure, is an important issue in the future.
DISCLOSURE
The authors declare that they have no competing interests. This manuscript is an original contribution, not previously published, and not under consideration for publication elsewhere.
Supplementary Material
ACKNOWLEDGMENTS
These studies were supported, in part, by grants from the National Natural Science Foundation of China (31730043, 31430043), National Key R&D Program of China (2018YFC1312700), Program of Innovative Research Team by National Natural Science Foundation (81721001), and the National Institutes of Health (5R01DK039308-31, 5R01HL092196-10, 5P01HL074940-13).
REFERENCES
- 1. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19•1 million participants. Lancet 2017; 389:37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Miguel C, Rudemiller NP, Abais JM, Mattson DL. Inflammation and hypertension: new understandings and potential therapeutic targets. Curr Hypertens Rep 2015; 17:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schiffrin EL. Immune mechanisms in hypertension and vascular injury. Clin Sci (Lond) 2014; 126:267–274. [DOI] [PubMed] [Google Scholar]
- 4. Yang T, Xu C. Physiology and pathophysiology of the intrarenal renin-angiotensin system: an update. J Am Soc Nephrol 2017; 28:1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Santos RAS, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M, Campagnole-Santos MJ. The ACE2/Angiotensin-(1-7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1-7). Physiol Rev 2018; 98:505–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/Angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ Res 2016; 118:1313–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu P, Costa-Goncalves AC, Todiras M, Rabelo LA, Sampaio WO, Moura MM, Santos SS, Luft FC, Bader M, Gross V, Alenina N, Santos RA. Endothelial dysfunction and elevated blood pressure in MAS gene-deleted mice. Hypertension 2008; 51:574–580. [DOI] [PubMed] [Google Scholar]
- 8. Rabelo LA, Xu P, Todiras M, Sampaio WO, Buttgereit J, Bader M, Santos RA, Alenina N. Ablation of angiotensin (1-7) receptor Mas in C57Bl/6 mice causes endothelial dysfunction. J Am Soc Hypertens 2008; 2:418–424. [DOI] [PubMed] [Google Scholar]
- 9. Rabello Casali K, Ravizzoni Dartora D, Moura M, Bertagnolli M, Bader M, Haibara A, Alenina N, Irigoyen MC, Santos RA. Increased vascular sympathetic modulation in mice with Mas receptor deficiency. J Renin Angiotensin Aldosterone Syst 2016; 17:1470320316643643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Moura MM, dos Santos RA, Campagnole-Santos MJ, Todiras M, Bader M, Alenina N, Haibara AS. Altered cardiovascular reflexes responses in conscious Angiotensin-(1-7) receptor Mas-knockout mice. Peptides 2010; 31:1934–1939. [DOI] [PubMed] [Google Scholar]
- 11. Collister JP, Nahey DB. Simultaneous administration of Ang(1-7) or A-779 does not affect the chronic hypertensive effects of angiotensin II in normal rats. J Renin Angiotensin Aldosterone Syst 2010; 11:99–102. [DOI] [PubMed] [Google Scholar]
- 12. van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 2018; 19:213–228. [DOI] [PubMed] [Google Scholar]
- 13. Todorova D, Simoncini S, Lacroix R, Sabatier F, Dignat-George F. Extracellular vesicles in angiogenesis. Circ Res 2017; 120:1658–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malloci M, Perdomo L, Veerasamy M, Andriantsitohaina R, Simard G, Martínez MC. Extracellular vesicles: mechanisms in human health and disease. Antioxid Redox Signal 2019; 30:813–856. [DOI] [PubMed] [Google Scholar]
- 15. Yin M, Loyer X, Boulanger CM. Extracellular vesicles as new pharmacological targets to treat atherosclerosis. Eur J Pharmacol 2015; 763:90–103. [DOI] [PubMed] [Google Scholar]
- 16. El Harane N, Kervadec A, Bellamy V, Pidial L, Neametalla HJ, Perier MC, Lima Correa B, Thiébault L, Cagnard N, Duché A, Brunaud C, Lemitre M, Gauthier J, Bourdillon AT, Renault MP, Hovhannisyan Y, Paiva S, Colas AR, Agbulut O, Hagège A, Silvestre JS, Menasché P, Renault NKE. Acellular therapeutic approach for heart failure: in vitro production of extracellular vesicles from human cardiovascular progenitors. Eur Heart J 2018; 39:1835–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de la Cuesta F, Baldan-Martin M, Moreno-Luna R, Alvarez-Llamas G, Gonzalez-Calero L, Mourino-Alvarez L, Sastre-Oliva T, López JA, Vázquez J, Ruiz-Hurtado G, Segura J, Vivanco F, Ruilope LM, Barderas MG. Kalirin and CHD7: novel endothelial dysfunction indicators in circulating extracellular vesicles from hypertensive patients with albuminuria. Oncotarget. 2017; 8:15553–15562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Felder RA, White MJ, Williams SM, Jose PA. Diagnostic tools for hypertension and salt sensitivity testing. Curr Opin Nephrol Hypertens 2013; 22:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, Sun F, Lu J, Yin Y, Cai X, Sun Q, Wang K, Ba Y, Wang Q, Wang D, Yang J, Liu P, Xu T, Yan Q, Zhang J, Zen K, Zhang CY. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell 2010; 39:133–144. [DOI] [PubMed] [Google Scholar]
- 20. Fraga-Silva RA, Ferreira AJ, Dos Santos RA. Opportunities for targeting the angiotensin-converting enzyme 2/angiotensin-(1-7)/mas receptor pathway in hypertension. Curr Hypertens Rep 2013; 15:31–38. [DOI] [PubMed] [Google Scholar]
- 21. Raffai G, Durand MJ, Lombard JH. Acute and chronic angiotensin-(1-7) restores vasodilation and reduces oxidative stress in mesenteric arteries of salt-fed rats. Am J Physiol Heart Circ Physiol 2011; 301:H1341–H1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hartmann D, Fiedler J, Sonnenschein K, Just A, Pfanne A, Zimmer K, Remke J, Foinquinos A, Butzlaff M, Schimmel K, Maegdefessel L, Hilfiker-Kleiner D, Lachmann N, Schober A, Froese N, Heineke J, Bauersachs J, Batkai S, Thum T. MicroRNA-Based therapy of GATA2-deficient vascular disease. Circulation 2016; 134:1973–1990. [DOI] [PubMed] [Google Scholar]
- 23. Scott DW, Vallejo MO, Patel RP. Heterogenic endothelial responses to inflammation: role for differential N-glycosylation and vascular bed of origin. J Am Heart Assoc 2013; 2:e000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cai J, Han Y, Ren H, Chen C, He D, Zhou L, Eisner GM, Asico LD, Jose PA, Zeng C. Extracellular vesicle-mediated transfer of donor genomic DNA to recipient cells is a novel mechanism for genetic influence between cells. J Mol Cell Biol 2013; 5:227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nazarenko I, Rupp AK, Altevogt P. Exosomes as a potential tool for a specific delivery of functional molecules. Methods Mol Biol 2013; 1049:495–511. [DOI] [PubMed] [Google Scholar]
- 26. Ribeiro D, Horvath I, Heath N, Hicks R, Forslöw A, Wittung-Stafshede P. Extracellular vesicles from human pancreatic islets suppress human islet amyloid polypeptide amyloid formation. Proc Natl Acad Sci USA 2017; 114:11127–11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Freed JK, Durand MJ, Hoffmann BR, Densmore JC, Greene AS, Gutterman DD. Mitochondria-regulated formation of endothelium-derived extracellular vesicles shifts the mediator of flow-induced vasodilation. Am J Physiol Heart Circ Physiol 2017; 312:H1096–H1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Valeri N, Braconi C, Gasparini P, Murgia C, Lampis A, Paulus-Hock V, Hart JR, Ueno L, Grivennikov SI, Lovat F, Paone A, Cascione L, Sumani KM, Veronese A, Fabbri M, Carasi S, Alder H, Lanza G, Gafa’ R, Moyer MP, Ridgway RA, Cordero J, Nuovo GJ, Frankel WL, Rugge M, Fassan M, Groden J, Vogt PK, Karin M, Sansom OJ, Croce CM. MicroRNA-135b promotes cancer progression by acting as a downstream effector of oncogenic pathways in colon cancer. Cancer Cell 2014; 25:469–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zeng C, Wang D, Yang Z, Wang Z, Asico LD, Wilcox CS, Eisner GM, Welch WJ, Felder RA, Jose PA. Dopamine D1 receptor augmentation of D3 receptor action in rat aortic or mesenteric vascular smooth muscles. Hypertension 2004; 43:673–679. [DOI] [PubMed] [Google Scholar]
- 30. Wang X, Wang J, Luo H, Chen C, Pei F, Cai Y, Yang X, Wang N, Fu J, Xu Z, Zhou L, Zeng C. Prenatal lipopolysaccharide exposure causes mesenteric vascular dysfunction through the nitric oxide and cyclic guanosine monophosphate pathway in offspring. Free Radic Biol Med 2015; 86:322–330. [DOI] [PubMed] [Google Scholar]
- 31. Peiró C, Vallejo S, Gembardt F, Azcutia V, Heringer-Walther S, Rodríguez-Mañas L, Schultheiss HP, Sánchez-Ferrer CF, Walther T. Endothelial dysfunction through genetic deletion or inhibition of the G protein-coupled receptor Mas: a new target to improve endothelial function. J Hypertens 2007; 25:2421–2425. [DOI] [PubMed] [Google Scholar]
- 32. Vrijenhoek JE, Pasterkamp G, Moll FL, de Borst GJ, Bots ML, Catanzariti L, van de Weg SM, de Kleijn DP, Visseren FL, Ruijter HM; SMART study group . Extracellular vesicle-derived CD14 is independently associated with the extent of cardiovascular disease burden in patients with manifest vascular disease. Eur J Prev Cardiol 2015; 22:451–457. [DOI] [PubMed] [Google Scholar]
- 33. Xu R, Greening DW, Zhu HJ, Takahashi N, Simpson RJ. Extracellular vesicle isolation and characterization: toward clinical application. J Clin Invest 2016; 126:1152–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li L, Zhu D, Huang L, Zhang J, Bian Z, Chen X, Liu Y, Zhang CY, Zen K. Argonaute 2 complexes selectively protect the circulating microRNAs in cell-secreted microvesicles. PLoS One 2012; 7:e46957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang JM, Su C, Wang Y, Huang YJ, Yang Z, Chen L, Wu F, Xu SY, Tao J. Elevated circulating endothelial microparticles and brachial-ankle pulse wave velocity in well-controlled hypertensive patients. J Hum Hypertens 2009; 23:307–315. [DOI] [PubMed] [Google Scholar]
- 36. Preston RA, Jy W, Jimenez JJ, Mauro LM, Horstman LL, Valle M, Aime G, Ahn YS. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension 2003; 41:211–217. [DOI] [PubMed] [Google Scholar]
- 37. Osada-Oka M, Shiota M, Izumi Y, Nishiyama M, Tanaka M, Yamaguchi T, Sakurai E, Miura K, Iwao H. Macrophage-derived exosomes induce inflammatory factors in endothelial cells under hypertensive conditions. Hypertens Res 2017; 40:353–360. [DOI] [PubMed] [Google Scholar]
- 38. You D, Loufrani L, Baron C, Levy BI, Widdop RE, Henrion D. High blood pressure reduction reverses angiotensin II type 2 receptor-mediated vasoconstriction into vasodilation in spontaneously hypertensive rats. Circulation 2005; 111:1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tanoue A, Nasa Y, Koshimizu T, Shinoura H, Oshikawa S, Kawai T, Sunada S, Takeo S, Tsujimoto G. The alpha(1D)-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J Clin Invest 2002; 109:765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1-7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension 2007; 49:185–192. [DOI] [PubMed] [Google Scholar]
- 41. Chen Y, Song YX, Wang ZN. The microRNA-148/152 family: multi-faceted players. Mol Cancer 2013; 12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sun X, Sit A, Feinberg MW. Role of miR-181 family in regulating vascular inflammation and immunity. Trends Cardiovasc Med 2014; 24:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gu Q, Wang B, Zhang XF, Ma YP, Liu JD, Wang XZ. Contribution of renin-angiotensin system to exercise-induced attenuation of aortic remodeling and improvement of endothelial function in spontaneously hypertensive rats. Cardiovasc Pathol 2014; 23:298–305. [DOI] [PubMed] [Google Scholar]
- 44. Rodrigues Prestes TR, Rocha NP, Miranda AS, Teixeira AL, Simoes-E-Silva AC. The anti-inflammatory potential of ACE2/Angiotensin-(1-7)/Mas receptor axis: evidence from basic and clinical research. Curr Drug Targets 2017; 18:1301–1313. [DOI] [PubMed] [Google Scholar]
- 45. Jiang F, Yang J, Zhang Y, Dong M, Wang S, Zhang Q, Liu FF, Zhang K, Zhang C. Angiotensin-converting enzyme 2 and angiotensin 1-7: novel therapeutic targets. Nat Rev Cardiol 2014; 11:413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li S, Zhu J, Zhang W, Chen Y, Zhang K, Popescu LM, Ma X, Lau WB, Rong R, Yu X, Wang B, Li Y, Xiao C, Zhang M, Wang S, Yu L, Chen AF, Yang X, Cai J. Signature microRNA expression profile of essential hypertension and its novel link to human cytomegalovirus infection. Circulation 2011; 124:175–184. [DOI] [PubMed] [Google Scholar]
- 47. Kontaraki JE, Marketou ME, Zacharis EA, Parthenakis FI, Vardas PE. MicroRNA-9 and microRNA-126 expression levels in patients with essential hypertension: potential markers of target-organ damage. J Am Soc Hypertens 2014; 8:368–375. [DOI] [PubMed] [Google Scholar]
- 48. Gildea JJ, Carlson JM, Schoeffel CD, Carey RM, Felder RA. Urinary exosome miRNome analysis and its applications to salt sensitivity of blood pressure. Clin Biochem 2013; 46:1131–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. DuPont JJ, McCurley A, Davel AP, McCarthy J, Bender SB, Hong K, Yang Y, Yoo JK, Aronovitz M, Baur WE, Christou DD, Hill MA, Jaffe IZ. Vascular mineralocorticoid receptor regulates microRNA-155 to promote vasoconstriction and rising blood pressure with aging. JCI Insight 2016; 1:e88942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kohlstedt K, Trouvain C, Boettger T, Shi L, Fisslthaler B, Fleming I. AMP-activated protein kinase regulates endothelial cell angiotensin-converting enzyme expression via p53 and the post-transcriptional regulation of microRNA-143/145. Circ Res 2013; 112:1150–1158. [DOI] [PubMed] [Google Scholar]
- 51. Zheng L, Xu CC, Chen WD, Shen WL, Ruan CC, Zhu LM, Zhu DL, Gao PJ. MicroRNA-155 regulates angiotensin II type 1 receptor expression and phenotypic differentiation in vascular adventitial fibroblasts. Biochem Biophys Res Commun 2010; 400:483–488. [DOI] [PubMed] [Google Scholar]
- 52. Zhu N, Zhang D, Chen S, Liu X, Lin L, Huang X, Guo Z, Liu J, Wang Y, Yuan W, Qin Y. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis 2011; 215:286–293. [DOI] [PubMed] [Google Scholar]
- 53. Karolina DS, Tavintharan S, Armugam A, Sepramaniam S, Pek SL, Wong MT, Lim SC, Sum CF, Jeyaseelan K. Circulating miRNA profiles in patients with metabolic syndrome. J Clin Endocrinol Metab 2012; 97:E2271–E2276. [DOI] [PubMed] [Google Scholar]
- 54. Fernandes T, Hashimoto NY, Magalhães FC, Fernandes FB, Casarini DE, Carmona AK, Krieger JE, Phillips MI, Oliveira EM. Aerobic exercise training-induced left ventricular hypertrophy involves regulatory MicroRNAs, decreased angiotensin-converting enzyme-angiotensin ii, and synergistic regulation of angiotensin-converting enzyme 2-angiotensin (1-7). Hypertension 2011; 58:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Goyal R, Goyal D, Leitzke A, Gheorghe CP, Longo LD. Brain renin-angiotensin system: fetal epigenetic programming by maternal protein restriction during pregnancy. Reprod Sci 2010; 17:227–238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.