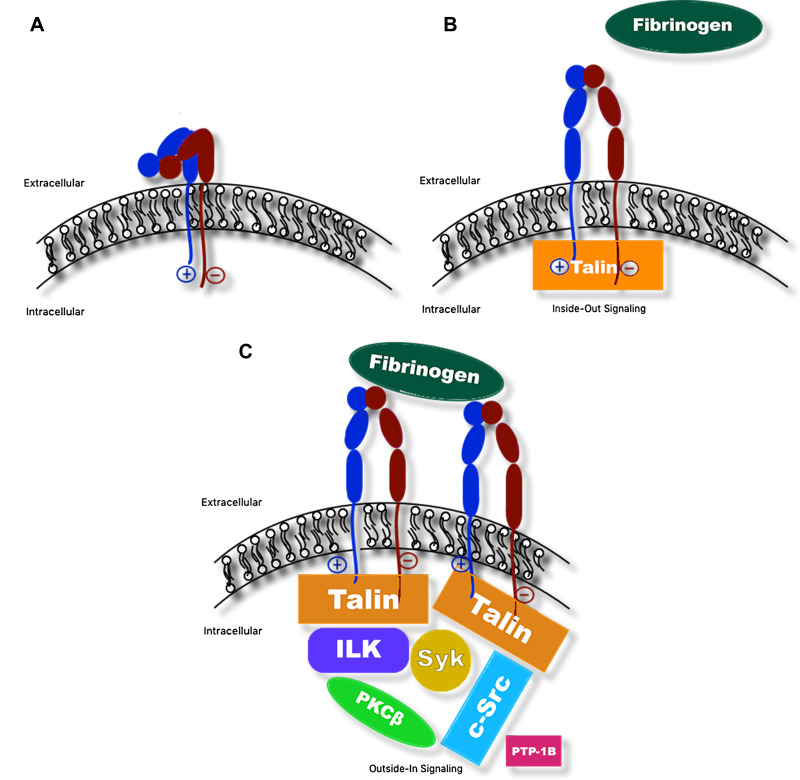
Figure 2.
Schematic of αIIbβ3 integrin undergoing inside-out and outside-in signaling. (A) Bent confirmation of αIIbβ3 integrin with intact salt bridge linking cytosolic domains of the subunits (low affinity for binding fibrinogen). (B) Binding of intracellular protein talin disrupts salt bridge and triggers separation of the cytosolic region of β3 from that of αIIb, resulting in a conformational change of the αIIbβ3 integrin into the upright position. In this position, fibrinogen is able to bind extracellular domains (high affinity for binding fibrinogen; inside-out signaling). (C) Fibrinogen, in turn, binds additional αIIbβ3 integrins to facilitate platelet aggregation, resulting in activation and recruitment of additional intracellular and cytosolic proteins, such as c-Src tyrosine kinase (c-Src), integrin-linked kinase (ILK), spleen tyrosine kinase (Syk), protein kinase C (PKC), and protein tyrosine phosphatase (PTP1B) and others, to facilitate processes including cytoskeletal reorganization for platelet spreading, clot stabilization, and clot retraction (outside-in signaling).