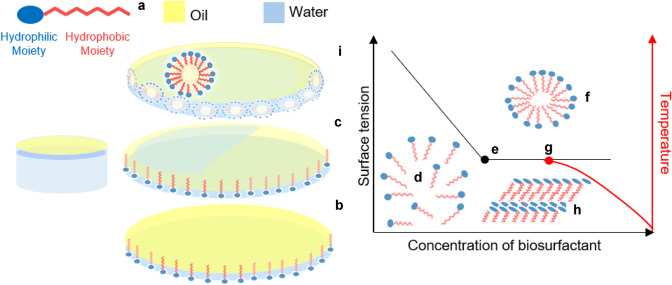
Fig. 1.
Biosurfactants are amphiphilic molecules, their composition consists of hydrophilic (carboxylic acids, alcohols, amino acids, phosphates, peptides, mono-, di- or polysaccharide cations or anions) and hydrophobic (hydrocarbon chains or saturated/unsaturated fatty acids) moieties (a). These molecules accumulate at phase interfaces of different polarities and stabilise heterogeneous phases (oil/air bubble droplets). b Thus, adsorption reduces the free energy per unit area needed to create a new surface, a reduction that is closely related to surface tension (liquid–air) and interfacial tension (liquid–liquid) [note the displacement of surface oil over water in c] [34]. Biosurfactant monomers (d) due to their self-assembly properties on reaching the Critical Micellar Concentration (CMC) (e), can form aggregated micellar structures (f) [35]. Micelle formation and solubility occur just above the Krafft temperature (g), i.e., the critical temperature at which micellar self-assembly occurs due to dissolution of the hydrated surfactant crystals (h) [36]. Bioemulsifiers are less effective in reducing surface tension and are involved in the formation and stabilization of emulsions between two immiscible phases (i) [31, 32]