Abstract
Objective: This research aimed at probing into miR-93-5p and miR-18a’s diagnostic and prognostic values in non-small cell lung cancer (NSCLC) patients. Methods: A total of 107 patients diagnosed with NSCLC in the Department of Oncology and Thoracic Surgery of our hospital from January 2015 to June 2016 were regarded as the research group (RG), and 42 healthy people were considered as the control group (CG). Serum samples were collected and miR-93-5p, miR-18a expression was detected via qPCR. The relationship between miR-93-5p, miR-18a and clinicopathological characteristics of NSCLC patients was assessed, and the diagnostic value of the two miRNAs was analyzed by ROC curve. Results: miR-93-5p and miR-18a were up-regulated in NSCLC. The higher the degree of tumor differentiation, the higher the TNM stage and the expression of the two miRNAs were. The high expression was tied to tumor differentiation degree, TNM stage, lymph node metastasis and lymph-vascular space invasion (LVSI). The survival rate of miR-93-5p and miR-18a high expression patients was worse than that of those with low expression. The AUC value of both of the mRNAs in NSCLC diagnosis was high (0.8905). Conclusion: The expression of miR-93-5p and miR-18a is associated with NSCLC severity and prognosis, and both can be used as potential markers for diagnosis.
Keywords: Non-small cell lung cancer, miR-93-5p, miR-18a, diagnostic value, prognosis analysis
Introduction
Worldwide, lung cancer (LC) is one of the main reasons for cancer-related deaths. The morbidity of LC has increased dramatically. It ranks first among all kinds of tumors in males, and those in females have also increased. Compared with other tumors like breast cancer (BC) or prostate cancer (PC), LC has a higher mortality [1]. At present, LC is mainly divided into two subtypes: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). NSCLC is the most common LC type, accounting for 85% of all LC [2]. It is a multi-factorial malignancy, which has many risk factors (smoking history, asbestos exposure and malnutrition) that all aggravate morbidity [3]. NSCLC patients have many treatment options, such as surgery, chemotherapy or radiotherapy [4]. Because NSCLC is often diagnosed late, it has drug resistance to cytotoxicity and lacks feasible and reliable early biomarkers, and the overall 5-year survival rate is still very low [5]. Hence, identifying new diagnostic biomarkers or therapeutic targets is vital to control tumor development.
microRNAs (miRNAs) are a kind of endogenous non-coding RNA involved in many physiological and pathological processes [6]. Because miRNA can provide gene diagnosis and treatment of LC and other tumors as a new potential target, it has been a research hotspot recently as a new generation of molecular targeted markers and therapy for tumors. Many studies have confirmed that there are marked differences in miRNA expression between LC and adjacent normal tissues. Twenty-seven different kinds of miRNA expressed in LC tissues were down-regulated by 2 fold compared with those in adjacent normal tissues [7]. The miR-205 expression in LC tissues of NSCLC patients also increased, and the level in LC tissues and serum of NSCLC and SCLC patients is increased compared with normal lung tissues and serum [8]. Recent research has shown that abnormal expression of miR-150 in NSCLC promotes tumor cell development [9].
miR-93-5p pertains to a miR-106b-25 gene cluster and it is a collateral that is homologous to the miR-17-92 proto-oncogene cluster [10]. However, miR-18a belongs to the miR-17-92 family [11]. The former is confirmed to have differential regulation in many cancers, such as liver cancer [12], lung adenocarcinoma [13], BC [14], head cancer and neck squamous cell carcinoma [15]. Normally, miR-18a expression changes in many physiological and pathological processes, during cell proliferation, apoptosis, epithelial-mesenchymal transition (EMT), tumorigenesis, tumor invasion and metastasis, etc. [16]. To explore the expression of both miRNAs in NSCLC patients and their diagnostic and prognostic values, this research detected the expression in serum of patients, tracked their survival within 3 years, and discussed the application of the two miRNAs in predicting NSCLC.
Data and methods
Research subjects
From January 2015 to June 2016, 107 NSCLC patients diagnosed in the Department of Oncology and Thoracic Surgery of Affiliated Tumor Hospital of Nantong University were selected and disease was confirmed by clinical manifestation, laboratory examination and pathology/cytology; including 59 males and 48 females. They were (60.3±12.7) years old on average. Among them, 45 cases were poorly differentiated, 32 were moderately differentiated, and 30 were highly differentiated. As to TNM stage, there were 33 cases in stage I, 49 in stage II, and 25 in stage III. In addition, 42 healthy patients in the same time period were enrolled into control group, including 24 males and 18 females. They were (61.5±11.8) years old on average. There was no marked difference in age, gender and other general data between both groups, which were comparable. All patients and healthy people were informed of this study. This research was approved by the Hospital Ethics Committees, and all subjects signed the informed consent form. Patients were followed up for 3 years by telephone contact or door-to-door visit, and their physical condition and life and death after treatment were recorded. The survival time was calculated on a monthly basis. Serum samples were voluntarily provided by all patients to analyze the miR-93-5p and miR-18a mRNA expression.
qPCR
All patients and healthy people had blood drawn on an empty stomach in the morning. Then, it was put in a 10 mL sterile blood collection vessel, let stand for 30 min at room temperature. Afterwards, it was centrifuged for 10 min at 4500 r/min. The serum was separated, and miR-93-5p and miR-18a mRNA’s concentration and purity were determined. Total RNA was assessed by TRIzol reagent (Invitrogen, USA) under aseptic and RNase-free conditions according to the instructions. Whether the total RNA was degraded was identified by agarose gel electrophoresis, and the concentration and purity were detected by Nano Drop (Thermo Scientific). The primers for miR-93-5p and miR-18a were designed and synthesized by Shanghai Sangon Biotechnology Co., Ltd. (Table 1). PrimeScript miRNA cDNA synthesis kit (Takara, Japan) reversed cDNA, and ABI 7500 fluorescence quantitative PCR instrument (Applie Biosystems, USA) was used for qPCR amplification. PCR parameters were as follows: pre-denaturation at 95°C for 5 min, denaturation at 95°C for 15 s, annealing at 65°C for 15 s, extension at 72°C for 32 s, amplification for 40 cycles. U6 was regarded as the internal reference, and 2-Ct method was used for standardization.
Table 1.
Primer sequence
| Upstream primer 5’-3’ | Downstream primer 5’-3’ | |
|---|---|---|
| miR-93-5p | ACACTCCAGCTGGGTCCT | CTCAACTGGTGTCGTGGA |
| GTACTGACGTGCCC | ||
| miR-18a | GGTAAGGTGCATCTAGTG | GACTGTTCCTCTCTTCCTC |
| U6 | GCTTCGGCAGCACATATACTAAAAT | CGCTTCACGAATTTGCGTGTCAT |
Statistical analysis
The above index data were input into SPSS 21.0 for statistical analysis and GraphPad Prism 8.0 for illustrations. The measurement data were expressed by Mean ± SD, and the data comparison methods between groups were independent-samples T test, Log rank test and one-way analysis of variance, and post hoc pairwise comparison adopted LSD-t test. The counting data were expressed by n and compared via χ2 test. Serum miR-93-5p and miR-18a’s diagnostic value in NSCLC patients was evaluated by receiver operating characteristic (ROC) curve. The survival situation was analyzed by Kaplan-Meier algorithm.
Results
miR-93-5p, miR-18a expression in NSCLC patients
In this paper, 107 NSCLC patients and 42 healthy people were selected, and the miR-93-5p and miR-18a expression in serum was tested via qPCR. The expression of miR-93-5p and miR-18a in NSCLC patients was remarkably higher than that of normal persons. Furthermore, the expression of the two was compared in patients with different differentiation degrees and TNM stages; the higher the differentiation degree, the higher the expression was. In TNM stage, levels of both miRNAs in stages I, II, III patients increased gradually, and the differences were statistically significant (P < 0.001) (Figure 1).
Figure 1.
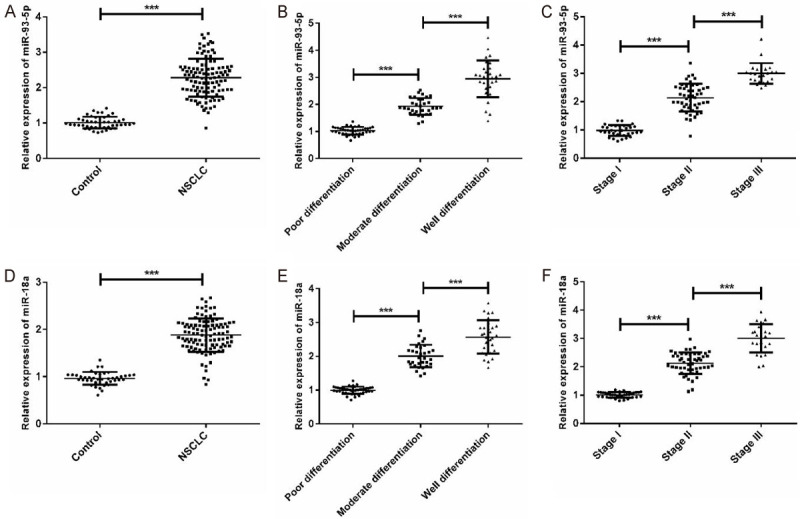
Expression of miR-93-5p and miR-18a in NSCLC patients. A: miR-93-5p is up-regulated in NSCLC patients; B: miR-93-5p expression in different differentiation degrees; C: miR-93-5p expression in patients with different TNM stages; D: miR-18a was up-regulated in NSCLC patients; E: miR-18a expression in different differentiation degrees; F: miR-18a expression in patients with different TNM stages; *** means P < 0.001.
Relationship between miR-93-5p, miR-18a expression and patients’ pathological characteristics
To clarify the clinical value of the miRNAs in NSCLC diagnosis and prognosis, this paper statistically analyzed the relationship between the two miRNAs and NSCLC patients’ clinicopathological features. In the light of miR-93-5p relative expression, the patients with a median above (2.29) were in the high expression group, and the rest were in the low expression group. Similarly, the median relative expression of miR-18a (1.88) was classified. It showed that both miRNA’s high expression was related to tumor differentiation degree, TNM stage, lymph node metastasis and lymph-vascular space invasion (LVSI) (Table 2).
Table 2.
Relationship between miR-93-5p, miR-18a expression and pathological features of patients
| n | miR-93-5p | X2 | P | miR-18a | X2 | P | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| High | Low | High | Low | ||||||
| Gender | 0.0022 | 0.9623 | 0.1905 | 0.6625 | |||||
| Male | 59 | 31 | 28 | 27 | 32 | ||||
| Female | 48 | 25 | 23 | 24 | 24 | ||||
| Age | 0.0149 | 0.9029 | 0.0764 | 0.7822 | |||||
| < 60 | 43 | 23 | 20 | 22 | 21 | ||||
| ≥ 60 | 64 | 35 | 29 | 31 | 33 | ||||
| Maximal tumor diameter | 0.0879 | 0.7668 | 0.5467 | 0.4597 | |||||
| < 5 cm | 63 | 39 | 24 | 34 | 29 | ||||
| ≥ 5 cm | 34 | 20 | 14 | 21 | 13 | ||||
| Differentiation degree | 6.5062 | 0.0387 | 7.5802 | 0.0226 | |||||
| Poorly differentiated | 45 | 18 | 27 | 16 | 29 | ||||
| Moderately differentiated | 32 | 17 | 15 | 18 | 14 | ||||
| Highly differentiated | 30 | 21 | 9 | 20 | 10 | ||||
| TNM stage | 6.5342 | 0.0381 | 6.3112 | 0.0426 | |||||
| I | 33 | 15 | 18 | 14 | 19 | ||||
| II | 49 | 33 | 16 | 32 | 17 | ||||
| III | 25 | 19 | 6 | 18 | 7 | ||||
| Lymph node metastasis | 5.2581 | 0.0218 | 5.4521 | 0.0195 | |||||
| Yes | 21 | 17 | 4 | 15 | 6 | ||||
| No | 86 | 40 | 46 | 37 | 49 | ||||
| Lymph-vascular space invasion | 5.1251 | 0.0236 | 5.3681 | 0.0205 | |||||
| Yes | 59 | 41 | 18 | 39 | 20 | ||||
| No | 48 | 23 | 25 | 21 | 27 | ||||
Prognosis analysis of miR-93-5p and miR-18a on the prognosis of NSCLC patients
The 3-year follow-up showed that the 3-year survival rate of high miR-93-5p expression patients was clearly lower than that of those with low expression (log-rank: P=0.0442), and the rate of high miR-18a expression patients was also lower (log-rank: P=0.0282) (Figure 2).
Figure 2.
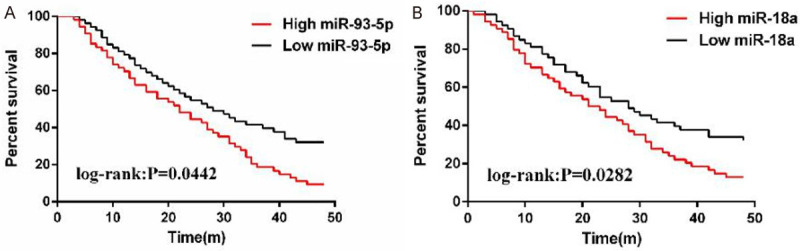
Prognosis analysis of miR-93-5p and miR-18a on prognosis of NSCLC patients. A: 4-year survival analysis of patients with high and low expression of miR-93-5p; B: 4-year survival analysis of patients with high and low expression of miR-18a.
miR-93-5p and miR-18a’s diagnostic value in NSCLC
In order to confirm whether miR-93-5p or miR-18a can be used as markers for NSCLC diagnosis, the ROC curves of the two were constructed. A total of 107 NSCLC patients were diagnosed by miR-93-5p, miR-18a and miR-93-5p+miR-18a. miR-93-5p’s AUC value in diagnosing NSCLC was 0.7962, the value of miR-18a was 0.8144, and that of miR-93-5p+miR-18a was 0.8905. The above results indicate that miR-93a+miR-18a has the best diagnostic value (Figure 3; Table 3).
Figure 3.
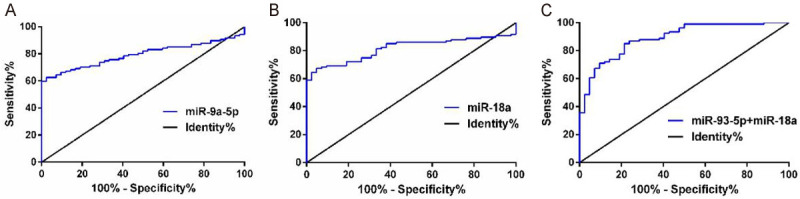
miR-93-5p and miR-18a for NSCLC diagnosis. A: Diagnostic value of miR-93-5p for NSCLC; B: Diagnostic value of miR-18a for NSCLC; C: Diagnostic value of miR-93-5p+miR-18a in NSCLC.
Table 3.
Diagnostic value of miR-93-5p and miR-18a for NSCLC
| AUC | S.E | 95% CI | P | |
|---|---|---|---|---|
| miR-93-5p | 0.7926 | 0.035 | 0.7231-0.8621 | < 0.0001 |
| miR-18a | 0.8144 | 0.034 | 0.7475-0.8813 | < 0.0001 |
| miR-93-5p+miR-18a | 0.8905 | 0.028 | 0.8357-0.9454 | < 0.0001 |
Discussion
Similar to tumor cells, the expression profile of miRNA in blood will also undergo characteristic changes, and these specific miRNA expression profiles can constitute the “fingerprint” of diseases and become a new noninvasive diagnostic marker, which is helpful for disease prediction, diagnosis and prognosis [17]. In tumor diseases, many miRNAs can be used as biomarkers because of their unique expression. For example, miR-155 can be used as a potential biomarker for detecting LC [18]; tumor suppressor gene miR-33a-5p/miR-128-3p in whole blood can be used as a predictor of early LC [19].
In this paper, the miR-93-5p and miR-18a levels in serum of NSCLC patients were measured by qPCR, and the relationship between the levels and NSCLC patients’ clinicopathological features was analyzed. The results revealed that both were up-regulated in NSCLC. The higher the degree of tumor differentiation, the higher the TNM stage and the expression of the two. The high expression was tied to pathological features. The survival rate of high expression patients was lower than that of those with low expression, and the levels were relevant to NSCLC. Recent research has shown that miR-93-5p pertains to a miR-106b-25 gene cluster, and its chromosomes are located at 7q22, which can promote G1/S phase transition of tumor cell’s cell cycle and proliferation and inhibit apoptosis [20]. miR-93-5p can also inhibit PTEN and RB1 through 3’-UTR which directly binds PTEN and RB1 mRNA. PTEN is a crucial tumor suppressor gene, which can act as a regulator of PKB/AKT/IKK signal transduction in the translation process. RB1 is a key tumor suppressor gene, which functions in cell cycle regulation. Both participate in many biological processes, thus activating NSCLC proliferation and metastasis [21]. Clinically, the miR-18a expression is relevant to many pathological features, and miR-18a up-regulation is negatively related to NSCLC efficacy; this may because it reduces the sensitivity of cells to radiation through activating the serine/threonine-protein kinase 4 (STK4) pathway [22]. At the same time, it was also discovered that miR-18a over-expression accelerated proliferation, migration and autophagy, and decreased apoptosis by inhibiting the expression of interferon regulatory factor 2 (IRF2) in LC [23]. In view of miR-93-5p and miR-18a’s molecular mechanism in LC and their influence on NSCLC prognosis, this paper holds that the high expression of the two miRNAs in NSCLC may be the result of stress response in the human body to NSCLC injury, so the severity of NSCLC in patients can be analyzed and their living conditions can be predicted based on their levels.
miR-93-5p, miR-18a and miR-93-5p+miR-18a’s ROC curves in NSCLC diagnosis were analyzed. It revealed that their AUC value in NSCLC diagnosis was the highest (0.8905), which indicated that the combined diagnosis of the two had better potential value in NSCLC. Ulivi Paola et al. [24] and Xu et al. [25] confirmed that both could be used as biomarkers for NSCLC diagnosis. According to the results of previous studies and this research, miR-93-5p+miR-18a have potential value in NSCLC diagnosis. There are also some shortcomings to this study. First of all, the sample size is small, and increasing the size can make the data conclusion more convincing. Secondly, the expression of miR-93-5p and miR-18a in patients’ serum can be further studied. For instance, research on mechanisms may be helpful, so follow-up trials will further explore the mechanism of miR-93-5p and miR-18a in patients.
To sum up, this paper studied miR-93-5p, miR-18a expression in NSCLC, and discussed their influence on prognosis. Both are highly expressed in serum of NSCLC patients, which is related to NSCLC pathological features. The 3-year prognosis of miR-93-5p and miR-18a over-expression group is poor. miR-93-5p+miR-18a has better diagnostic value. Thus, both can be used as potential NSCLC markers for diagnosis and prognosis analysis.
Disclosure of conflict of interest
None.
References
- 1.Nasim F, Sabath BF, Eapen GA. Lung cancer. Med Clin North Am. 2019;103:463–473. doi: 10.1016/j.mcna.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Liu G, Pei F, Yang F, Li L, Amin AD, Liu S, Buchan JR, Cho WC. Role of autophagy and apoptosis in non-small-cell lung cancer. Int J Mol Sci. 2017;18:367. doi: 10.3390/ijms18020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grieshober L, Graw S, Barnett MJ, Thornquist MD, Goodman GE, Chen C, Koestler DC, Marsit CJ, Doherty JA. Methylation-derived neutrophil-to-lymphocyte ratio and lung cancer risk in heavy smokers. Cancer Prev Res (Phila) 2018;11:727–734. doi: 10.1158/1940-6207.CAPR-18-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visconti R, Morra F, Guggino G, Celetti A. The between now and then of lung cancer chemotherapy and immunotherapy. Int J Mol Sci. 2017;18:367. doi: 10.3390/ijms18071374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botticella A, Mezquita L, Le Pechoux C, Planchard D. Durvalumab for stage III non-small-cell lung cancer patients: clinical evidence and real-world experience. Ther Adv Respir Dis. 2019;13:1753466619885530. doi: 10.1177/1753466619885530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganju A, Khan S, Hafeez BB, Behrman SW, Yallapu MM, Chauhan SC, Jaggi M. miRNA nanotherapeutics for cancer. Drug Discov Today. 2017;22:424–432. doi: 10.1016/j.drudis.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao W, Yu Y, Cao H, Shen H, Li X, Pan S, Shu Y. Deregulated expression of miR-21, miR-143 and miR-181a in non small cell lung cancer is related to clinicopathologic characteristics or patient prognosis. Biomed Pharmacother. 2010;64:399–408. doi: 10.1016/j.biopha.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Duan B, Guo T, Sun H, Cai R, Rui Q, Xi Z. miR-205 as a biological marker in non-small cell lung cancer. Biomed Pharmacother. 2017;91:823–830. doi: 10.1016/j.biopha.2017.04.086. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Ouyang R, Wang Z, Zhou W, Chen H, Jiang Y, Zhang Y, Li H, Liao M, Wang W, Ye M, Ding Z, Feng X, Liu J, Zhang B. MiR-150 promotes cellular metastasis in non-small cell lung cancer by targeting FOXO4. Sci Rep. 2016;6:39001. doi: 10.1038/srep39001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S, Chen X, Sun KX, Xiu YL, Liu BL, Feng MX, Sang XB, Zhao Y. MicroRNA-93 promotes epithelial-mesenchymal transition of endometrial carcinoma cells. PLoS One. 2016;11:e0165776. doi: 10.1371/journal.pone.0165776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao CH, Cheng GC, He RL, Hong Y, Wan QL, Wang ZZ, Pan ZY. Analysis and clinical significance of microRNA-499 expression levels in serum of patients with acute myocardial infarction. Genet Mol Res. 2015;14:4027–4034. doi: 10.4238/2015.April.27.17. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Liao Z, Bai Z, He Y, Duan J, Wei L. MiR-93-5p promotes cell proliferation through down-regulating PPARGC1A in hepatocellular carcinoma cells by bioinformatics analysis and experimental verification. Genes (Basel) 2018;9:51. doi: 10.3390/genes9010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Liu S, Mao Y, Xu J, Yang S, Shen H, Xu W, Fan W, Wang J. CircRNF13 regulates the invasion and metastasis in lung adenocarcinoma by targeting miR-93-5p. Gene. 2018;671:170–177. doi: 10.1016/j.gene.2018.04.069. [DOI] [PubMed] [Google Scholar]
- 14.Xiang Y, Liao XH, Yu CX, Yao A, Qin H, Li JP, Hu P, Li H, Guo W, Gu CJ, Zhang TC. MiR-93-5p inhibits the EMT of breast cancer cells via targeting MKL-1 and STAT3. Exp Cell Res. 2017;357:135–144. doi: 10.1016/j.yexcr.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, He Y, Liu C, Li G, Lu S, Jing Q, Chen X, Ma H, Zhang D, Wang Y, Huang D, Tan P, Chen J, Zhang X, Liu Y, Qiu Y. miR-93-5p enhances migration and invasion by targeting RGMB in squamous cell carcinoma of the head and neck. J Cancer. 2020;11:3871–3881. doi: 10.7150/jca.43854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen K, Cao Z, Zhu R, You L, Zhang T. The dual functional role of MicroRNA-18a (miR-18a) in cancer development. Clin Transl Med. 2019;8:32. doi: 10.1186/s40169-019-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Backes C, Meese E, Keller A. Specific miRNA disease biomarkers in blood, serum and plasma: challenges and prospects. Mol Diagn Ther. 2016;20:509–518. doi: 10.1007/s40291-016-0221-4. [DOI] [PubMed] [Google Scholar]
- 18.Shao C, Yang F, Qin Z, Jing X, Shu Y, Shen H. The value of miR-155 as a biomarker for the diagnosis and prognosis of lung cancer: a systematic review with meta-analysis. BMC Cancer. 2019;19:1103. doi: 10.1186/s12885-019-6297-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan J, Zhou C, Zhao X, He J, Tian H, Shen W, Han Y, Chen J, Fang S, Meng X, Jin X, Gong Z. A two-miRNA signature (miR-33a-5p and miR-128-3p) in whole blood as potential biomarker for early diagnosis of lung cancer. Sci Rep. 2018;8:16699. doi: 10.1038/s41598-018-35139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brett JO, Renault VM, Rafalski VA, Webb AE, Brunet A. The microRNA cluster miR-106b~25 regulates adult neural stem/progenitor cell proliferation and neuronal differentiation. Aging (Albany NY) 2011;3:108–124. doi: 10.18632/aging.100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang W, Bai J, Liu D, Wang S, Zhao N, Che R, Zhang H. MiR-93-5p up-regulation is involved in non-small cell lung cancer cells proliferation and migration and poor prognosis. Gene. 2018;647:13–20. doi: 10.1016/j.gene.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Shen Z, Wu X, Wang Z, Li B, Zhu X. Effect of miR-18a overexpression on the radiosensitivity of non-small cell lung cancer. Int J Clin Exp Pathol. 2015;8:643–648. [PMC free article] [PubMed] [Google Scholar]
- 23.Liang C, Zhang X, Wang HM, Liu XM, Zhang XJ, Zheng B, Qian GR, Ma ZL. MicroRNA-18a-5p functions as an oncogene by directly targeting IRF2 in lung cancer. Cell Death Dis. 2017;8:e2764. doi: 10.1038/cddis.2017.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ulivi P, Petracci E, Marisi G, Baglivo S, Chiari R, Billi M, Canale M, Pasini L, Racanicchi S, Vagheggini A, Delmonte A, Mariotti M, Ludovini V, Bonafe M, Crino L, Grignani F. Prognostic role of circulating miRNAs in early-stage non-small cell lung cancer. J Clin Med. 2019;8:131. doi: 10.3390/jcm8020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X, Zhu S, Tao Z, Ye S. High circulating miR-18a, miR-20a, and miR-92a expression correlates with poor prognosis in patients with non-small cell lung cancer. Cancer Med. 2018;7:21–31. doi: 10.1002/cam4.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]


