Abstract
Objective: This study was designed to demonstrate the accuracy of ultra-high performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) in detecting serum concentration of anti-schizophrenic drugs in patients with mental illness. Methods: The study participants were 186 schizophrenia patients treated in our hospital. Serum concentrations of anti-schizophrenic drugs in Chinese patients with mental illness were evaluated according to the reference intervals of drug therapy recommended in the guidelines. Results: Five drugs, namely Aripiprazole (ARI), Amisulpride (AMI), Olanzapine (OLA), Paliperidone (PAL) and Ziprasidone (ZIP) all showed good linearity within the linear range. The within-day precision of the above five drugs was all between 1.3%-8.9% and the inter-day precision was between 1.8%-7.6%, with within-day and inter-day relative standard deviations (RSDs) less than 15.00% and accuracy ranging from 87.00% to 106.73%. However, AMI had a mean blood concentration of 436.31±241.05 ng/mL (median concentration: 379.34 ng/mL), which was significantly higher than the reference range (100-320 ng/mL) recommended in the guidelines. Good recovery rates (86.21%-99.77%) were obtained after the samples were stored at room temperature for 24 h, at 4°C for 48h and at -20°C for half a year. Conclusions: Given that UPLC-MS/MS renders more accurate results in detecting the concentration of psychotropic drugs, it can be applied clinically to detect the concentration of therapeutic drugs in patients with mental illness.
Keywords: Ultra-high performance liquid chromatography-tandem mass spectrometry, schizophrenia, drug concentration
Introduction
Schizophrenia, currently one of the most serious mental diseases in the world, is a psychopathological form that includes disorders in perception, emotion and thinking [1]. The main cause is the loss of the basic functions of the brain such as ventricular enlargement and gray matter atrophy, resulting in varying degrees of mental activity disorders [2]. The initial manifestations of the disease are significant personality changes, as well as abnormal and uncontrolled emotions, accompanied with common symptoms of severe disorders in consciousness, memory and daily life, leading to patients’ strange behaviors or performances that are incomprehensible to ordinary people [3]. Currently, drug therapy is the mainstay of clinical intervention of psychiatric patients. However, most patients require long-term maintenance treatment with drugs, which leads to delayed healing of some patients and increases the risk of other diseases [4]. Moreover, literature has shown that long-term use of anti-psychotropic drugs in patients with mental illness will increase their psychological and biological drug dependence, which is not conducive to the control of their conditions. Therefore, it is of paramount importance to reduce drug addiction and dependence in patients with mental illness and to find more drugs with better therapeutic effect and fewer side effects [5,6]. However, there are currently few detection methods for drug concentration in the blood of patients with schizophrenia in China, which affects the treatment schedule and plan. In view of this, the ultra-high performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method was adopted to detect the concentration of Aripiprazole (ARI), Amisulpride (AMI), Olanzapine (OLA), Paliperidone (PAL) and Ziprasidone (ZIP) in the serum of patients with schizophrenia, so as to provide an effective basis for the selection of drugs in the treatment of this disease.
Materials and methods
General materials
This study was approved by the Medical Ethics Committee of our hospital. The selected samples for this trial were 186 schizophrenic patients treated in our hospital from February 2018 to May 2019, of whom, the ratio of male (100 cases) to female (86 cases) was 50:43; the age was 35-63 years old, with the average age of (48.2±6.8) years; and the course of disease ranged from 2 to 10 years with an average course of (4.58±1.46) years.
Inclusion and exclusion criteria
Inclusion criteria: Each enrolled patient met the ICD-10 criteria for schizophrenia symptoms, with the informed consent signed by the guardian(s). Exclusion criteria: Patients with severe somatic diseases that affected antipsychotic drug metabolism; patients who dropped out of this trial; patients with incomplete clinical data.
Instruments and reagents
Waters Acquity UPLC-TQ-D LC/MS system (Waters, USA); H1650R desktop high-speed refrigerated centrifuge (Shanghai Luxiangyi Centrifuge Instrument Co., Ltd., China); BT125D electronic balance (Sartorius, Germany); G560E vortex mixer (Scientific Industries, USA); formic acid (chromatographic grade, Shanghai Aladdin, Co. Ltd., China, Cat. No. F112034-100mL); chromatographic methanol (Merck, Germany); Watsons distilled water (Guangzhou Watsons’s Food & Beverage Co., Ltd., China).
Blood collection
Patients were given a single full dose at a fixed time every night. After 5 days, blood samples were collected at a uniform time in the morning. Briefly, venous blood (2 mL) was collected from each patient into vacuum blood collection tubes (yellow cap, 5 mL) where separation gel and coagulant were added, and centrifuged at 3,500 r/min for 3 min. Then the obtained serum was stored at -20°C.
Working solution ratio
The analyte and its stable isotope labeled internal standard (SIL-IS) stock solution were prepared at a concentration of 100 μg/mL in methanol and stored at -20°C. The standard curve and quality control serum of the corresponding concentration were obtained by using the control serum as the solvent dilution reserve solution [7] (Table 1).
Table 1.
Standard curve concentration and quality control products (High-QC, Medium-QC, Low-QC)
| Drug name | Standard curve concentration (ng/mL) | SIL-IS (ng/mL) | High-QC (ng/mL) | Medium-QC (ng/mL) | Low-QC (ng/mL) |
|---|---|---|---|---|---|
| ARI | 10.0-1280.0 ng/mL | 100.0 ng/mL | 1000.0 ng/mL | 500.0 ng/mL | 25.0 ng/mL |
| AMI | 20.0-2560.0 ng/mL | 100.0 ng/mL | 2000.0 ng/mL | 500.0 ng/mL | 50.0 ng/mL |
| OLA | 2.5-320.0 ng/mL | 20.0 ng/mL | 250.0 ng/mL | 50.0 ng/mL | 5.0 ng/mL |
| PAL | 2.5-320.0 ng/mL | 20.0 ng/mL | 250.0 ng/mL | 50.0 ng/mL | 5.0 ng/mL |
| ZIP | 5.0-640.0 ng/mL | 20.0 ng/mL | 500.0 ng/mL | 100.0 ng/mL | 10.0 ng/mL |
Note: SIL-IS: stable isotope labeled internal standard; ARI: Aripiprazole; AMI: Amisulpride; OLA: Olanzapine; PAL: Paliperidone; ZIP: Ziprasidone.
Conditions of chromatography and mass spectrometry
Acquity UPLC BEH C18 IVD (2.1 mm × 50 mm, 1.7 μm) chromatographic column was used, with 0.1% aqueous formic acid solution as mobile phase A and 0.1% formic acid-methanol solution as mobile phase B. Gradient elution was performed as follows: 0-0.8 min 5% B, 0.8-1.3 min 5%-80% B, 1.3-1.6 min 80%-95% B, 1.6-1.65 min 95-5% B, 1.65-2 min 5% B, with a flow rate of 0.5 mL/min. The positive ion mode and multiple-reaction monitoring mode of electrospray ionization (ESI) source were adopted for mass spectrometry detection [8]. The mass spectrum parameters of five drugs and their corresponding SIL-ISs are shown in Table 2.
Table 2.
Mass spectrometry parameters of drugs and corresponding SIL-ISs
| Drug name | Precursor ion (m/z) | Product ion (m/z) | De-clustering potential DP (V) | Collision pressure CE (eV) |
|---|---|---|---|---|
| ARI | 448.03 | 285.10 | 54 | 26 |
| ARI-d8 | 456.03 | 293.15 | 54 | 26 |
| AMI | 370.07 | 241.97 | 54 | 26 |
| AMI-d5 | 375.08 | 241.98 | 52 | 27 |
| OLA | 313.03 | 256.05 | 48 | 22 |
| OLA-d3 | 316.07 | 256.06 | 42 | 22 |
| PAL | 427.10 | 207.11 | 50 | 28 |
| PAL-d4 | 430.90 | 211.04 | 52 | 28 |
| ZIP | 412.97 | 194.03 | 54 | 30 |
| ZIP-d8 | 420.99 | 193.90 | 56 | 30 |
Note: SIL-IS: stable isotope labeled internal standard; ARI: Aripiprazole; AMI: Amisulpride; OLA: Olanzapine; PAL: Paliperidone; ZIP: Ziprasidone.
Linear equation and linear range
The standard curve was calculated with the peak area ratio of analyte and SIL-IS as the ordinate and the concentration of analyte in the serum as the abscissa to obtain the regression equation.
Lowest limit of quantification
A series of standard serum samples with a mass concentration of 0.02-25 ng/mL-1 of each compound were determined under chromatographic conditions. The minimum concentration of standard serum samples meeting the requirements of accuracy and precision at the same time was the lowest limit of quantification (LLOQ), namely, the lowest linearity point.
Precision and accuracy
Five quality control samples were prepared from blank human serum, and determined for 3 days to calculate the corresponding mass concentration. Meanwhile, within-day and inter-day precision and accuracy were investigated respectively.
Recovery and matrix effect
Three groups of samples were prepared to examine the matrix effects: in Group 1, a mixed solution of analyte and SIL-IS was prepared; in Group 2, analyte and SIL-IS were added to the blank serum samples after pre-treatment, and; in Group 3, analyte and SIL-IS were added to the blank serum samples before pre-treatment. The MF value was obtained by comparing the peak areas of the analytes in Group 2 and Group 1, while the SIL-IS normalized MF was obtained by comparing the peak area ratios of the analytes and SIL-ISs in Groups 2 and 1. For the experimental results of different human serum matrix, the CV should not be higher than 15% [9].
Stability investigation
After storage at room temperature (25°C) for 24 h, ordinary refrigeration temperature (4°C) for 48 d and -20°C for half a year, the sample stock solution was collected for recovery test to determine the stability under different storage conditions.
Statistical analysis
All the data were analyzed by SPSS 23.00 statistical software. The measurement results were represented by mean ± standard deviation (x̅ ± sd), and analyzed by the independent sample t test.
Results
Linear equation and linear range
The linear ranges of ARI, AMI, OLA, PAL and ZIP were 10.0-1280.0 ng/mL, 20.0-2560.0 ng/mL, 2.5-320.0 ng/mL, 2.5-320.0 ng/mL, and 5.0-640.0 ng/mL, respectively, and the correlation coefficients (r2) were 0.998849, 0.999161, 0.999334, 0.999545, and 0.997643, respectively, indicating that the five drugs had good linearity within the linear range (Table 3).
Table 3.
Linear equation and linear range
| Drug name | Linear range (ng) | Linear equation | Correlation coefficients (r2) |
|---|---|---|---|
| ARI | 10.0-1280.0 | y = 0.0085412x-0.00391624 | 0.998849 |
| AMI | 20.0-2560.0 | y = 0.00978084x-0.000522478 | 0.999161 |
| OLA | 2.5-320.0 | y = 0.05263x+0.00300725 | 0.999334 |
| PAL | 2.5-320.0 | y = 0.00722055x-0.000603362 | 0.999545 |
| ZIP | 5.0-640.0 | y = 0.00674121x-0.000306634 | 0.997643 |
Note: ARI: Aripiprazole; AMI: Amisulpride; OLA: Olanzapine; PAL: Paliperidone; ZIP: Ziprasidone.
Standard curve and lowest limit of quantification
ARI, AMI, OLA, PAL and ZIP all responded well at the lower limit of quantification of 1.0 μg/L (Figure 1).
Figure 1.
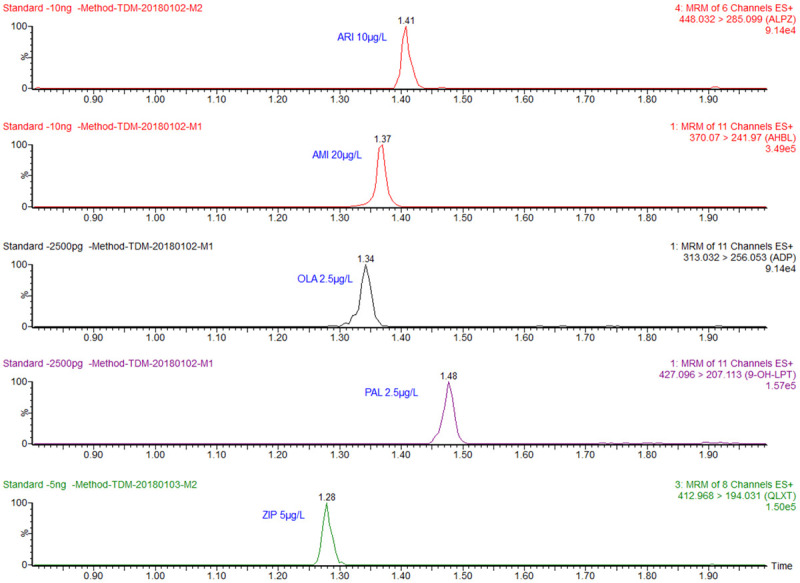
Drug response at the lower limit of quantification. ARI: Aripiprazole (10 μg/L); AMI: Amisulpride (20 μg/L); OLA: Olanzapine (2.5 μg/L); PAL: Paliperidone (2.5 μg/L); ZIP: Ziprasidone (5 μg/L).
Within-day and inter-day precision and accuracy
The results showed that the within-day precision and the inter-day precision of ARI, AMI, OLA, PAL and ZIP were 1.3%-8.9% and 1.8%-7.6% respectively. The within-day and inter-day relative standard deviations (RSDs) of all the five drugs were less than 15.00%, and the accuracy was 87.00%-106.73% (Table 4).
Table 4.
Within-day and inter-day precision and accuracy
| Analyte | Concentration (ng/mL) | Within-day | Inter-day | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Mean (ng/mL) | RSD (%) | Accuracy (%) | Mean (ng/mL) | RSD (%) | Accuracy (%) | ||
| ARI | 25.0 | 24.8 | 3.4 | 99.20 | 25.5 | 6.9 | 101.84 |
| 500.0 | 501.1 | 3.6 | 100.23 | 500.3 | 3.7 | 100.06 | |
| 1000.0 | 986.4 | 3.9 | 98.64 | 988.8 | 3.4 | 98.88 | |
| AMI | 50.0 | 46.4 | 3.9 | 92.74 | 47.1 | 3.7 | 94.12 |
| 500.0 | 474.3 | 2.4 | 94.87 | 474.4 | 2.2 | 94.88 | |
| 2000.0 | 1999.2 | 2.3 | 99.96 | 1975.1 | 1.8 | 98.76 | |
| OLA | 5.0 | 4.4 | 8.4 | 87.60 | 4.4 | 7.6 | 87.00 |
| 50.0 | 44.9 | 3.0 | 89.78 | 45.2 | 2.4 | 90.34 | |
| 250.0 | 248.8 | 1.3 | 99.53 | 251.3 | 2.7 | 100.52 | |
| PAL | 5.0 | 4.9 | 5.3 | 98.40 | 4.9 | 5.5 | 98.20 |
| 50.0 | 49.0 | 2.2 | 98.02 | 49.8 | 3.0 | 99.66 | |
| 250.0 | 260.1 | 1.8 | 104.04 | 266.8 | 3.2 | 106.73 | |
| ZIP | 10.0 | 9.9 | 8.9 | 98.60 | 9.6 | 6.4 | 96.00 |
| 100.0 | 98.8 | 4.1 | 98.80 | 99.0 | 4.9 | 99.03 | |
| 500.0 | 505.4 | 1.7 | 101.07 | 505.6 | 2.6 | 101.11 | |
Note: RSD: relative standard deviation; ARI: Aripiprazole; AMI: Amisulpride; OLA: Olanzapine; PAL: Paliperidone; ZIP: Ziprasidone.
Recovery and matrix effect
The results revealed that the matrix effects of ARI, AMI, OLA, PAL and ZIP were all in the range of 0.94-1.17, with small fluctuation, which can be considered as no matrix effect (Table 5).
Table 5.
Recovery and matrix effect
| Analyte | Concentration (ng/mL) | Recovery (%) | Matrix effect |
|---|---|---|---|
| ARI | 25.0 | 105.87 | 1.05 |
| 500.0 | 98.93 | 0.95 | |
| 1000.0 | 100.97 | 1.00 | |
| AMI | 50.0 | 100.40 | 1.11 |
| 500.0 | 99.10 | 0.98 | |
| 2000.0 | 96.82 | 1.10 | |
| OLA | 5.0 | 101.07 | 1.06 |
| 50.0 | 105.88 | 1.17 | |
| 250.0 | 104.57 | 0.96 | |
| PAL | 5.0 | 102.67 | 1.01 |
| 50.0 | 98.77 | 0.94 | |
| 250.0 | 98.74 | 0.98 | |
| ZIP | 10.0 | 98.80 | 0.95 |
| 100.0 | 102.06 | 1.08 | |
| 500.0 | 103.32 | 0.94 |
Note: ARI: Aripiprazole; AMI: Amisulpride; OLA: Olanzapine; PAL: Paliperidone; ZIP: Ziprasidone.
Drug concentration monitoring results
Compared with the treatment reference range, the detection results of ARI, OLA, PAL and ZIP all showed good consistency, indicating that the recommended reference range of these four drugs in the guidelines also had good applicability in China. However, for AMI, the mean blood concentration was 436.31±241.05 ng/mL (median concentration: 379.34 ng/mL), which was significantly higher than the reference range of 100-320 ng/mL recommended in the guidelines (Table 6).
Table 6.
Drug concentration monitoring results
| Analyte | Concentration range (ng/mL) | Median (ng/mL) | Mean (ng/mL) | Reference range (ng/mL) |
|---|---|---|---|---|
| ARI | 20.47-723.64 | 245.39 | 249.67±130.14 | 150-500 |
| AMI | 78.18-945.34 | 379.34 | 436.31±241.05 | 100-320 |
| OLA | 12.89-143.62 | 42.16 | 48.37±19.47 | 20-80 |
| PAL | 13.24-86.17 | 34.97 | 38.16±21.47 | 20-60 |
| ZIP | 30.11-145.07 | 50.78 | 71.48±40.75 | 50-200 |
Note: ARI: Aripiprazole; AMI: Amisulpride; OLA: Olanzapine; PAL: Paliperidone; ZIP: Ziprasidone.
Stability investigation
Good recovery rates (86.21%-99.77%) were obtained after the samples were stored at room temperature for 24 h, at 4°C for 48 h and at -20°C for half a year (Table 7).
Table 7.
Concentration changes of drugs stored at 4°C and -20°C
| Analyte | Initial concentration (ng/mL) | Store at room temperature for 24 h (ng/mL) | Store at room temperature for 7 d (ng/mL) | 4°C refrigerator 48 h (ng/mL) | Store at -20°C (ng/mL) |
|---|---|---|---|---|---|
| ARI | 26.41±0.52 | 25.36±0.95 | 24.69±0.66 | 25.08±0.44 | 25.15±0.50 |
| 1058.72±25.93 | 1060.70±18.41 | 1016.43±19.57 | 1024.72±23.77 | 1017.68±23.81 | |
| AMI | 50.88±1.38 | 48.23±1.81 | 46.33±1.94 | 47.84±2.86 | 47.52±1.62 |
| 1931.25±48.59 | 1763.43±64.06 | 1658.69±34.25 | 1730.81±101.81 | 1768.95±46.48 | |
| OLA | 5.30±0.26 | 5.05±0.19 | 4.90±0.13 | 5.17±0.40 | 4.99±0.29 |
| 273.63±4.93 | 250.67±7.89 | 235.90±5.24 | 251.12±12.08 | 255.13±9.94 | |
| PAL | 4.76±0.06 | 4.66±0.12 | 4.47±0.12 | 4.45±0.14 | 4.45±0.15 |
| 244.73±3.22 | 237.83±4.70 | 231.79±2.43 | 230.81±4.30 | 227.78±1.71 | |
| ZIP | 11.48±0.24 | 11.45±0.25 | 10.59±0.30 | 10.89±0.29 | 11.00±0.21 |
| 532.32±7.82 | 490.65±7.18 | 494.97±7.46 | 494.14±8.70 | 491.66±5.00 |
Note: ARI: Aripiprazole; AMI: Amisulpride; OLA: Olanzapine; PAL: Paliperidone; ZIP: Ziprasidone.
Discussion
At present, therapeutic drug monitoring (TDM) for psychotropic drugs in China is still in its infancy and lacks systematic research. This gap will undoubtedly adversely affect the safety and efficacy of related drugs [10]. Due to the great difference in individual drug metabolism ability of adolescents and elderly patients, the serum concentration is prone to be high without TDM, resulting in toxic and side effects (dizziness, cognitive impairment, constipation, etc.). For patients with long-term out-of-hospital medication, TDM can help doctors to quickly and accurately understand the patient’s compliance with medical advice, and assist in the diagnosis of relapse caused by improper medication. To accurately quantify drug concentrations, a variety of analytical techniques have been introduced, including high performance liquid chromatography (HPLC), gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS) [11]. Although most of them yield results with sufficient sensitivity and accuracy, complex pre-treatments (such as extraction, drying) and long chromatographic separation time hinder their popularization in daily clinical TDM [12]. Recently, the emergence of UPLC-MS/MS has attracted wide attention.
The increasing application of UPLC-MS/MS in recent years is due to the excellent resolution of tandem mass spectrometry detector and the efficient separation capability of UPLC. Samples can be analyzed rapidly by simple pre-treatment such as protein precipitation, which makes UPLC-MS/MS a promising method for TDM in clinic [13]. Using the efficient and stable UPLC-MS/MS method established in this study, clinical samples in our hospital were examined [14]. It was found that ARI, OLA, PAL and ZIP show good consistency compared with the reference range of treatment, indicating that the recommended reference range of these four drugs in this guideline also has good applicability in China. On the other hand, it suggests that UPLC-MS/MS has better accuracy in detecting drug concentration in the blood of patients with mental illness, which can be extended to detection of other drugs and to monitoring of the concentration of anti-schizophrenic drugs in clinical practice. According to the test results and clinical feedback of nearly 500 samples, UPLC-MS/MS has shown the following advantages: (1) when the patient’s condition has not improved significantly after medication, it assists in evaluating the safety of increasing the dosage of drugs; (2) when the patient develops adverse reactions (drowsiness, vomiting, constipation, etc.), it can help to determine whether it is caused by excessive blood concentration; (3) it helps doctors confirm the previous medication of patients referred to hospitals; (4) it assists doctors to confirm the compliance of the patient with the prescribed medication during the out-of-hospital period [15,16]. It has been shown that through TDM, individualized drug delivery scheme can be realized, so as to improve drug therapy and achieve safe, effective and rational drug use in clinic [17]. Xin et al. determined that the mean blood concentration of 42 AMI specimens was significantly higher than the recommended reference range in the guidelines [18]. In view of the preceding research results, some researchers collected the clinical medication information of patients, some of which had AMI blood concentration exceeding the “AGNP laboratory warning level” (640 ng/mL). However, in the actual treatment process they found that: (1) under high AMI blood concentration levels, there were no obvious side effects in these patients; (2) when the clinician reduced the dosage of AMI, the disease relapsed [19]. Based on this, we speculate that the metabolic behavior and therapeutic effect of AMI drugs in Chinese population are quite different from those in European and American population, so it is necessary to determine the reference range of AMI treatment concentration suitable for the Chinese population through more systematic clinical trials and data accumulation in the later period. Such studies on racial differences in drug metabolism have also been found in other antipsychotics like Clozapine, but no relevant studies have been published on blood concentration of AMI in the Chinese [20,21]. Yang et al. established a highly applicable UPLCMS/MS method for the simultaneous quantitative determination of five antipsychotic drugs in plasma [22]. Their clinical test results revealed that the concentrations of ARI, OLA, PAL and ZIP accord with the therapeutic concentration reference range in AGNP guidelines; whereas for AMI, the serum concentration of patients is significantly higher than the recommended range. In order to achieve the goal of rational and safe drug use, redefining of the reference range of AMI treatment concentration for Chinese population is warranted through more systematic research [23].
Due to the small number of patients selected in this experiment and the small sample size, there is a certain deviation in the detection results. There are also many types of drugs available to treat schizophrenia in China, but not many were analyzed in this study. It is hoped that the concentration of more kinds of psychotropic drugs can be detected in future studies, so as to provide more evidences for the clinical treatment of patients with mental illness.
In conclusion, UPLC-MS/MS is more accurate in detecting the concentration of psychotropic drugs, and can be clinically used to detect the concentration of therapeutic drugs in patients with mental diseases.
Acknowledgements
This work was supported by the S&T Major Project of Lishui (2017ZDYF15) and the Foundation of China’s State Key Laboratory for Diagnosis and Treatment of Infectious Diseases. The authors thank all of the participants who recruited patients in this study.
Disclosure of conflict of interest
None.
References
- 1.Lieberman JA. Disease modifying effects of antipsychotic drugs in schizophrenia: a clinical and neurobiological perspective. World Psychiatry. 2018;17:163–165. doi: 10.1002/wps.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang X, Shen XD, Guo YY. The effect of olanzapine on body mass index, plasma leptin and hypothalamic neurohormones in patients with schizophrenia. China Pharm. 2018;21:843–846. [Google Scholar]
- 3.Dong DB, Wang YL, Chang XB, Luo C, Yao DZ. Dysfunction of large-scale brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Schizophr Bull. 2018;44:168–181. doi: 10.1093/schbul/sbx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knudsen HC, Vázquez-Barquero JL, Welcher B, Gaite L, Becker T, Chisholm D, Ruggeri M, Schene AH, Thornicroft G. Translation and cross-cultural adaptation of outcome measurements for schizophrenia: EPSILON study 2. Br J Psychiatry. 2018;37:2068–2075. doi: 10.1192/bjp.177.39.s8. [DOI] [PubMed] [Google Scholar]
- 5.Han SX, An ZG, Luo X, Zhang LL, Zhong XJ, Du W, Yi QZ, Shi YY. Association between CMYA5 gene polymorphisms and risk of schizophrenia in uygur population and a meta-analysis. Early Interv Psychiatry. 2018;12:685–691. doi: 10.1111/eip.12276. [DOI] [PubMed] [Google Scholar]
- 6.Li GN, Cui HM, Li D, Zhou YL, Sui B, Fan N. The effect of reducing the dosage of antipsychotic drugs on mental symptoms in stable schizophrenia. Chin J Nerv Ment Dis. 2018;44:24–28. [Google Scholar]
- 7.Liao RY, Zhang T, He YQ, Shen LJ, Sang DE, Xu CL. The effect of metacognitive training on social cognition and insight of schizophrenia patients. Chin J Nerv Ment Dis. 2018;44:32–37. [Google Scholar]
- 8.Humpston CS. The Paradoxical self: Awareness, solipsism and first-rank symptoms in schizophrenia. Philos Psychol. 2018;43:1–22. [Google Scholar]
- 9.Dumont M, Thériault J, Briand C, Dumais A, Potvin S. Psychosocial approaches for individuals with schizophrenia in correctional and forensic psychiatric settings: a rapid review. J Forensic Pract. 2018;20:1425–1429. [Google Scholar]
- 10.Cheng Y, Ju PJ, Cui DH. MicroRNA-137 gene and schizophrenia. Chin J Behav Med Brain Sci. 2018;34:85–88. [Google Scholar]
- 11.Liu XM, Chen R, Zeng GH, Gao Y, Liu XP, Zhang DL, Hu P, Wang HY, Jiang J. Determination of a PDE4 inhibitor hemay005 in human plasma and urine by UPLC-MS/MS and its application to a PK study. Bioanalysis. 2018;10:863–875. doi: 10.4155/bio-2018-0004. [DOI] [PubMed] [Google Scholar]
- 12.Yu WB, Xu CC, Li GM, Hong WP, Zhou ZY, Xiao CX, Zhao YQ, Cai YF, Huang M, Jin J. Simultaneous determination of trimethylamine N-oxide, choline, betaine by UPLC-MS/MS in human plasma: an application in acute stroke patients. J Pharm Biomed Anal. 2018;152:179–187. doi: 10.1016/j.jpba.2018.01.049. [DOI] [PubMed] [Google Scholar]
- 13.Wang HQ, Wang M, Luo Z, Qin YP, Nan F, Xiang J, Shu SQ, Zhu XH, Liang MZ, Yu Q, Ye LM. Determination of plasma concentration by UPLC-MS/MS and bioequivalence of two finasteride preparations in human. Chin J New Drugs. 2018;27:437–442. [Google Scholar]
- 14.Yang N, Sun TH, Huang FH, Li LY, Zhang XL, Li WY. Simultaneous determination of the content and pharmacokinetics of the main components of Shenqi Fuzheng injection in rat plasma by UPLC-MS/MS. Chin J Hosp Pharm. 2018;38:1250–1255. [Google Scholar]
- 15.Liang S, Yang Q, Li RL, Wang YH. Determination of aristolochic acid A in 13 Chinese patent medicines by UPLC-MS/MS. West Chin J Pharm Sci. 2020;35:82–85. [Google Scholar]
- 16.Zhang J, Yuan YL, Xiong W. UPLC-MS/MS method for determination of carbamazepine in human plasma. Chin J Drug Appl Monitor. 2020;17:26–29. [Google Scholar]
- 17.Cui C, Hu P, Jiang J, Kong FS, Luo H, Zhao Q. An UPLC-MS/MS method to determine CT-707 and its two metabolites in plasma of ALK-positive advanced non-small cell lung cancer patients. J Pharm Biomed Anal. 2018;153:1–8. doi: 10.1016/j.jpba.2018.01.042. [DOI] [PubMed] [Google Scholar]
- 18.Lan X, Gao HJ, Li YL, Chen B, Chen GR, Chen L. UPLC-MS/MS-based Pharmacokinetic study of harmine in different doses of single administration in rats. Chin J Clin Pharmacol. 2019;35:3095–3100. [Google Scholar]
- 19.Zhao ZX, Yang J, Li J, Zhang XB, Jin Y, Guo JQ, Guo LP. Simultaneous determination of 7 boswellic acids in medicinal boswellia by UPLC-MS/MS. World Chin Med. 2019;14:2855–2859. [Google Scholar]
- 20.Ma H, Ding CG, Fang BH, Kang XY, Ge QH. Simultaneous determination of enalapril and enalaprilat in human plasma by UPLC-MS/MS and its clinical application under fasting and fed conditions. Chin J Pharm. 2018;49:572–580. [Google Scholar]
- 21.Wang L, Yan T, Zhang KX, Li FF, Jia JM, Hu GS. A sensitive UPLC-MS/MS method for simultaneous determination of polyphenols and theaflavins in rat plasma: application to a pharmacokinetic study of Da Hong Pao Tea. Biomed Chromatogr. 2019;42:2452–2462. doi: 10.1002/bmc.4470. [DOI] [PubMed] [Google Scholar]
- 22.Yang X, Li L, He LJ, He LN, Lou Y, Lu XY. Establishment of UPLC-MS/MS analytical method for the concentration of lamotrigine, olanzapine and quetiapine in human plasma and monitoring of therapeutic drugs. Chin Pharm J. 2020;45:44–51. [Google Scholar]
- 23.Zuo LH, Hu YR, Zhou L, Sun Z, Jiang XF, Liu X, Kang J, Zhang XJ. UPLC-MS/MS method for determination of multiple active ingredients in Guanxinshutong capsules and drug quality evaluation. J Shenyang Pharm Univ. 2018;35:103–110. [Google Scholar]


