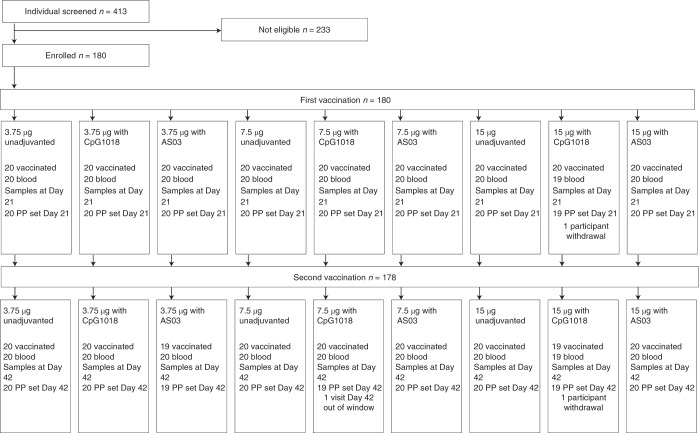
Fig. 1. Trial profile—participant disposition.
Enrollment and follow-up of study participants vaccinated with 3.75 µg, 7.5 µg or 15 µg CoVLP with or without AS03 or CpG1018 adjuvant after the first and second dose administration. One participant in the 3.75 µg + AS03 treatment group did not receive the second vaccination as per protocol owing to a grade 3 AE (fatigue) after the first dose but agreed to have blood collection for immunogenicity. One participant in the 15 µg + CpG1018 treatment group withdrew consent before the second vaccination and consequently did not have blood collection at Day 21 and Day 42. Both participants were excluded from the per-protocol set. For more details of participant disposition, see Table 1. PP, per-protocol.