Fig. 3. Humoral response to CoVLP alone or with adjuvants.
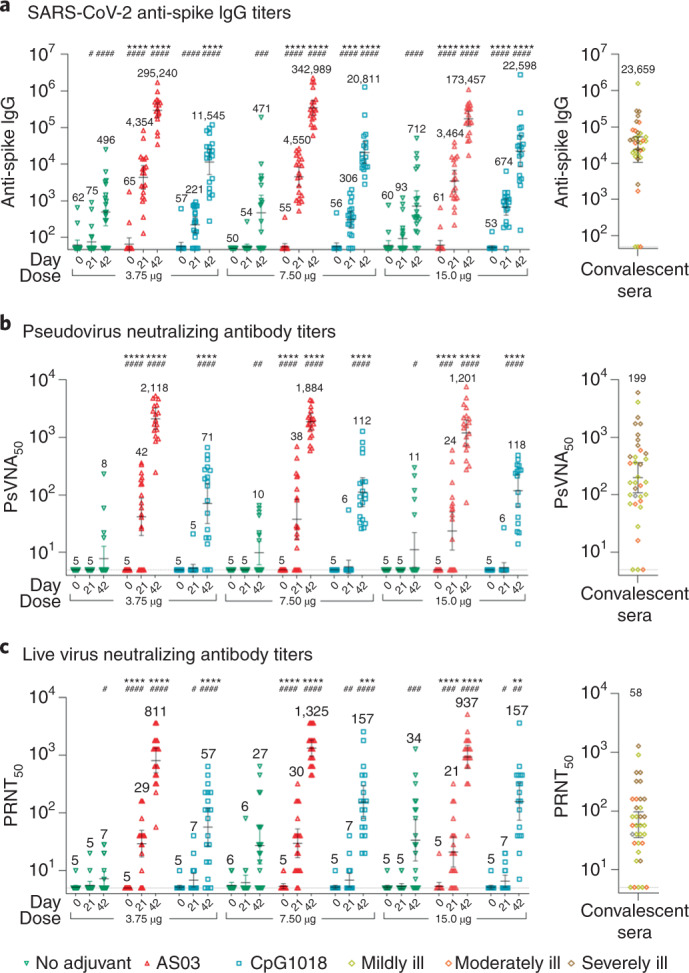
Serum antibodies of participants vaccinated with 3.75 µg, 7.5 µg or 15 µg CoVLP with or without AS03 or CpG1018 adjuvant were measured to S protein by ELISA (a) and in neutralization assays based on a VSV pseudovirus (b) or live virus (c) and presented here as reciprocal titers. Inverted green triangles are used for unadjuvanted CoVLP groups; upright red triangles are used for the CoVLP + AS03 groups; and blue squares are used for the CoVLP + CpG1018 groups. Convalescent sera or plasma were collected at least 14 d after a positive diagnosis of COVID-19 (RT–PCR) from individuals whose illness was classified as mild, moderate or severe/critical (n = 35). These samples were analyzed in the anti-S ELISA and both neutralization assays; results (right panels). Horizontal bars and numbers in the figure indicate geometric means. Error bars indicate 95% CIs. Significant differences among Days 0 and 21 or Days 0 and 42 for each formulation are indicated by a hashtag (#P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001; paired two-sided t-test of log-transformed values, GraphPad Prism v8.1.1). Significant differences between unadjuvanted and adjuvanted regimens for Days 21 and 42 are indicated by an asterisk (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; two-way ANOVA of log-transformed values, GraphPad Prism v8.1.1). The PsVNA50 is reciprocal of the serum dilution at which a decrease in luminescence ≥50% was observed in the PNA. The PRNT50 is the reciprocal serum dilution at which ≥50% of the cells were free from infection in the MNA.
