Abstract
Key message
Carbon isotope discrimination is a promising trait for indirect screening for improved water use efficiency of C4 crops.
Abstract
In the context of a changing climate, drought is one of the major factors limiting plant growth and yield. Hence, breeding efforts are directed toward improving water use efficiency (WUE) as a key factor in climate resilience and sustainability of crop production. As WUE is a complex trait and its evaluation is rather resource consuming, proxy traits, which are easier to screen and reliably reflect variation in WUE, are needed. In C3 crops, a trait established to be indicative for WUE is the carbon isotopic composition (δ13C) of plant material, which reflects the preferential assimilation of the lighter carbon isotope 12C over 13C during photosynthesis. In C4 crops, carbon fixation is more complex and δ13C thus depends on many more factors than in C3 crops. Recent physiological and genetic studies indicate a correlation between δ13C and WUE also in C4 crops, as well as a colocalization of quantitative trait loci for the two traits. Moreover, significant intraspecific variation as well as a medium to high heritability of δ13C has been shown in some of the main C4 crops, such as maize, sorghum and sugarcane, indicating its potential for indirect selection and breeding. Further research on physiological, genetic and environmental components influencing δ13C is needed to support its application in improving WUE and making C4 crops resilient to climate change.
Improved water use efficiency to mitigate for the effect of changing climatic conditions
Climate change comprises a variety of environmental changes, including increases in CO2 concentrations, temperatures and unstable precipitation (Hatfield and Dold 2019). Since these environmental factors have a strong influence on key plant processes, affecting both photosynthesis and water relations, plant performance needs to be optimized under new climatic conditions and limitations. Water deficit is one of the major factors impairing crop growth and yield (Leakey et al. 2019). Therefore, a main focus of improving the resilience of plants to the changing climatic conditions is increasing their water use efficiency (WUE) to enhance sustainability of agriculture, save water and contribute to food security (Condon et al. 2004; Leakey et al. 2019).
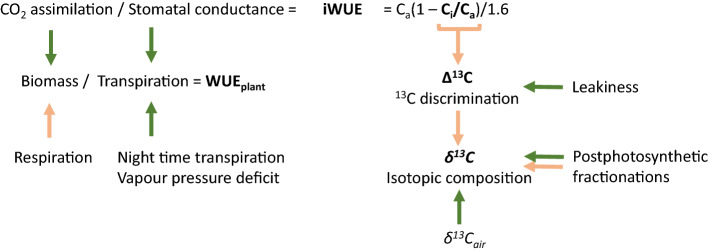
In the context of plant production, WUE is defined as the ratio of yield (grain or biomass) to water received or evapotranspired by the system (e.g., field plot, Ellsworth and Cousins 2016). In a more narrow sense, WUE at the single plant level (WUEplant) represents the amount of biomass produced per volume of water transpired. The main component of WUEplant is the intrinsic WUE (iWUE) at the leaf level, representing the ratio of CO2 assimilation rate to stomatal conductance (Fig. 1, Medrano et al. 2015). As both CO2 assimilation and stomatal conductance are influenced by several environmental and genetic factors, iWUE is a complex trait. In addition to iWUE, important components of WUEplant are the air water vapor pressure deficit, which is the difference between the amount of moisture in the air and the maximum air moisture at saturation, nighttime transpiration and carbon loss through respiration (Ellsworth et al. 2020). A high iWUE can either be achieved through an increase in CO2 assimilation rate without a corresponding increase in stomatal conductance or by reducing stomatal conductance without a corresponding decrease in CO2 assimilation rate (Leakey et al. 2019). In C4 crops, high assimilation rates can be realized at relatively low stomatal conductance due to the specific characteristics of the C4 cycle and its CO2 concentrating mechanism, leading to an elevated iWUE (Way et al. 2014). In severe drought, however, it has been shown, that the advantage in WUE of C4 grasses, such as maize (Zea mays L.), disappears (Blankenagel et al. 2018). Several studies have demonstrated significant genetic variation for WUE within C4 species and thus potential for further genetic improvement of WUE (Geetika et al. 2019; Hammer et al. 1997; Henderson et al. 1998; Jackson et al. 2016; Leakey et al. 2019; Ryan et al. 2016; Sinclair 2012; Xin et al. 2009).
Fig. 1.
Associations between water use efficiency (WUE) and the carbon isotopic composition of C4 plant material. Negative effects are depicted by light orange arrows, positive effects are depicted by dark green arrows. The WUE of a plant (WUEplant) can be assessed by the destructive measurement of biomass in relation to the sum of water transpired by the plant. The biomass, which the plant accumulates, depends on assimilation rate and respiration, while the water transpired by the plant depends on the stomatal conductance, as well as night time transpiration and the vapor pressure deficit of the air over its lifetime. The intrinsic WUE (iWUE) is defined as the ratio of assimilation rate over stomatal conductance of a leaf section at a specific time and is by definition related to the ratio of the intercellular CO2 concentration (Ci) to the ambient CO2 concentration (Ca; Yang et al. 2016). This ratio of Ci/Ca is theoretically negatively correlated to the discrimination against the 13C isotope during assimilation (∆13C), when the influence of leakiness is stable below 0.37 as it was observed, e.g., in Henderson et al. (1992). The isotopic composition of tissues like leaves and grains (δ13C) is an indirect and integrated measure for ∆13C, when the isotopic composition of the air (δ13Cair) is accounted for. Post-photosynthetic fractionations influence δ13C further as these fractionations lead to distinct isotopic signatures of different plant compounds, which through their relative contribution to the composition of a tissue determine its δ13C
Despite increased efforts focused on improving WUE, the complexity of the trait has restricted the breeding progress in this area (Chen et al. 2011). The high number of physiological factors determining WUE and the variability of the plants’ responses to different environments have impeded the use of traditional breeding methods directed at WUE improvement (Leakey et al. 2019). Additionally, the difficulty of high-throughput screening for WUE has been a major limiting factor. Screening of WUEplant requires gravimetric tracking of water uptake and destructive measurement of biomass production. Screening for iWUE with gas exchange measuring systems is also very time- and labor-intensive. Therefore, both WUEplant and iWUE are difficult to measure on large populations needed for successful breeding (Chen et al. 2011). Additionally, screening of WUE is typically performed in phenotyping platforms with controlled environmental conditions (Ryan et al. 2016) and it has often been difficult to translate the results to the performance under field conditions (Araus and Cairns 2014). Hence, the identification of proxy traits that are easy to measure on a large number of plants, and reliably reflect variation in WUE would greatly support advances in breeding for drought resistance (Chen et al. 2011; Leakey et al. 2019). In C3 plants, such a proxy trait is carbon isotope discrimination (Δ13C), which describes the preferential assimilation of the lighter carbon isotope 12C over the heavier 13C during the process of photosynthesis. The extent of this discrimination is dependent on the ratio of intercellular to ambient CO2 partial pressure (Ci/Ca), determined by CO2 assimilation rate and stomatal conductance. Since this dependence is shared with WUE, Δ13C is reflective of environmental conditions affecting CO2 assimilation, stomatal conductance and genotypic differences in WUE. When plants are grown under uniform environmental conditions, Δ13C has been established to be indicative for genotypic differences in WUE as well as yield under drought (Farquhar and Richards 1984; Saranga et al. 1998). Therefore, Δ13C has been applied in a breeding program and giving rise to more water use efficient wheat varieties (Condon et al. 2004).
For C4 species, the use of Δ13C as a proxy for WUE is less clear due to the more complex nature of carbon fixation and Δ13C compared to C3 species (Farquhar 1983). In addition to the ratio of CO2 assimilation rate and stomatal conductance, the leakage of CO2 from the bundle sheath cells back to the mesophyll determines Δ13C as an additional contributing factor (Fig. 1, Farquhar 1983). This leakage is affected by the coordination of different photosynthetic enzymes and influences the efficiency of photosynthesis. Therefore, in addition to studies focused on WUE, Δ13C is of high interest for studying limitations of photosynthetic efficiency, especially in response to changing environmental conditions (Kromdijk et al. 2014). Due to the difficulties of integrating all the abovementioned components, Δ13C research in C4 crops has not advanced as actively as in C3 plants. Only recently, due to the progress in phenotyping and genotyping technologies, there have been advances in our understanding of the factors influencing both Δ13C and WUE as well as their interconnectivity in C4 plants. For broadening our knowledge in this research area, the combination of genetic studies, identifying underlying quantitative trait loci (QTL) and the universality of their effects in different genetic backgrounds and environments, with physiological studies, unraveling the interaction of different Δ13C determinants and their environmental dependence, is needed.
This review will provide an overview of the current knowledge on carbon isotope discrimination in C4 plants in the context of breeding for enhanced water use efficiency.
Carbon isotope discrimination during carbon assimilation and its theoretical connection to WUE
Carbon naturally occurs as two stable isotopes, 12C and 13C, the latter of which is only present in 1.1% of CO2 in the atmosphere (Farquhar et al. 1989a). In plants, the 13C/12C ratio is even lower than in air, indicating that plants discriminate against the heavier isotope. This discrimination happens mainly during photosynthetic CO2 assimilation by the plant. The stable carbon isotopic composition of a sample, e.g. air or plant material, (δ13C) is conventionally expressed as the 13C/12C ratio of the sample (Rs) in reference to the 13C/12C ratio of the Pee Dee Belemnite Standard (RPDB), a fossil with an exceptionally high amount of the 13C isotope (Eq. (1), Farquhar et al. 1982).
| 1 |
This results in current values for δ13C in the air (δ13Cair) of about − 8.5 ‰, with a trend to decrease over the years due to the increase in anthropogenic emissions (Graven et al. 2017). The difference between the δ13C of the analyzed plant sample (δ13Cp; typically plant dry matter) and δ13Cair surrounding the plant is described by the carbon isotope discrimination (Δ13C) of plants (Eq. (2), Farquhar et al. 1982).
| 2 |
Due to the discrimination against 13C during carbon assimilation, δ13C of plant material shows more negative values than that of air. The average δ13C of C3 plant tissue is around − 28 ‰, corresponding to a Δ13C of 20 ‰ (Farquhar et al. 1989a). Δ13C during C3 photosynthesis is characterized primarily by the more frequent use of the 12C over 13C isotope by Rubisco (Ribulose-1,5-bisphosphate carboxylase/oxygenase), the main enzyme contributing to carbon fixation, owing to a lower reactivity of 13C. Additionally, several alterations in the 13C/12C ratio of CO2, called isotopic fractionations, occur during diffusion of CO2 from the atmosphere to the site of carbon fixation (Farquhar et al. 1982). Since these fractionation factors of Rubisco carboxylation and diffusion are relatively constant, in C3 plants a linear positive correlation between Δ13C and the ratio of intercellular (Ci) to ambient (Ca) CO2 partial pressure (Ci/Ca) is predicted and observed (Farquhar et al. 1989a). Ci/Ca, on the other hand, is determined mainly by stomatal conductance and photosynthetic capacity and thus directly connected to intrinsic water use efficiency. As a consequence, a strong inverse correlation between Δ13C and WUE can be expected in C3 plants. Several studies on a variety of C3 species, including important agricultural crops like wheat, barley, soybean, peanut, cotton, rice, potato and tomato, have confirmed this inverse relationship between WUE and Δ13C experimentally using both dry matter derived estimates (Barbour et al. 2010; Condon et al. 2004; Hubick and Farquhar 1989; Hubick et al. 1986; Impa et al. 2005; Martin et al. 1999; Saranga et al. 1998; Vos and Groenwold 1989) and short-term measurements (Evans et al. 1986) of Δ13C. These analyses build the foundation for the application of Δ13C in breeding for improved WUE of C3 plants.
In C4 species, the carbon concentrating mechanism is determined by the Kranz anatomy, locally separating the initial carbon fixation from the Rubisco-catalyzed CO2 assimilation in mesophyll and bundle sheath cells, respectively. This, in turn, leads to additional complexity of Δ13C (Farquhar 1983; von Caemmerer et al. 2014; Fig. 2). A comprehensive model of Δ13C of C4 plants is described by Farquhar and Cernusak (2012). A more simplified model of Δ13C as a function of leakiness (φ) and Ci/Ca is given by Eq. (3) (Farquhar 1983).
| 3 |
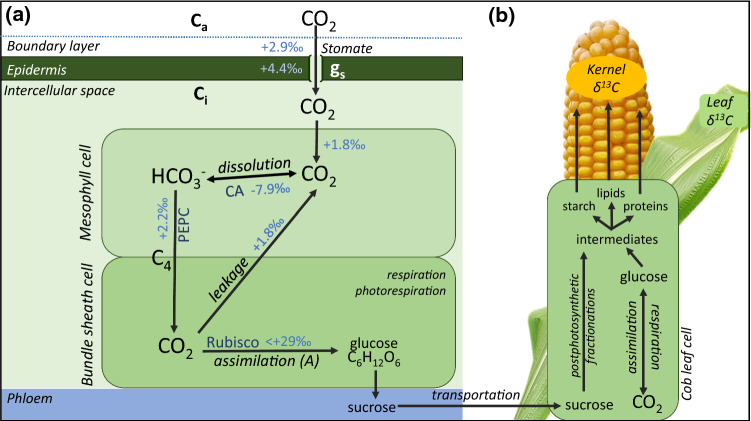
Fig. 2.
Simplified presentation of the factors influencing carbon isotope discrimination (a) and the resulting isotopic composition (δ13C) of leaves and grains (b) in C4 plants. a CO2 entering the leaf diffuses through the boundary layer and stomata (stomatal conductance gs), whereby discriminations against the 13C isotope (discrimination factors are shown in lighter blue and were reviewed by Ubierna et al. (2018b)) take place. Diffusion in the cytoplasm of mesophyll cells contributes further to discrimination against 13C, whereas there is an enrichment in 13C accompanying the conversion of CO2 to HCO3−, catalyzed by carbonic anhydrase (CA) and a relatively small discrimination during fixation by phosphoenolpyruvate carboxylase (PEPC). By active transportation via C4 dicarboxylic acids (malate or aspartate), CO2 is enriched in the bundle sheath cell. The discrimination realized by Ribulose-1,5-bisphosphate carboxylase/-oxygenase (Rubisco) depends on the leakage of CO2 back into the mesophyll, which itself comes with a discrimination factor. Additional factors influencing the discrimination during assimilation are respiration and photorespiration. For more details we refer the reader to an excellent review by von Caemmerer et al. (2014). The ratio between intercellular CO2 concentration (Ci) and ambient CO2 concentration (Ca), which determines the intrinsic water use efficiency, is correlated with the 13C discrimination. b) Assimilates, carrying an isotopic signature influenced by Ci/Ca during their assimilation can be transported, predominantly as sucrose, via the phloem and unloaded in sink tissues, where they contribute to the carbon isotopic composition of these tissues (δ13C). Additionally, the glucose assimilated in the tissue itself and other compounds like starch, lipids and proteins determine δ13C. Due to post-photosynthetic fractionations during their synthesis, starch, lipids and proteins carry distinct isotopic signatures. The relative composition of compounds of distinct isotopic signatures is likely to contribute to differences observed when measuring the isotopic composition in whole tissues of leaves and grains (grain δ13C, leaf δ13C)
Values for the fractionation factors, including the fractionation during diffusion of CO2 in air (a), in the liquid phase (s), Rubisco carboxylation (b3) and the combined fractionation of CO2 dissolution and PEPC carboxylation (b4) are reviewed by Ubierna et al. (2018b). After diffusion through the stomata (a = 4.4‰), CO2 is converted to bicarbonate by carbonic anhydrase in the mesophyll cells and subsequently fixed by the phosphoenolpyruvate carboxylase (PEPC). Due to fractionation during dissolution of CO2, the bicarbonate is enriched in 13C. Since the discrimination against 13C by PEPC is smaller than the enrichment during dissolution, there is an overall 13C enrichment during this initial fixation step to a C4 acid (b4 ≈ − 5.7 ‰, Farquhar 1983). The C4 acid is then transported to the bundle sheath cell and decarboxylated. The released CO2 is re-fixed by Rubisco. Here, the discrimination by Rubisco (b3 ≈ 29‰) depends on the leakage of some CO2 back to the mesophyll cell (Farquhar 1983). This leakage originates from the concentration gradient between the two cell types and is quantified by the leakiness, defined as the fraction of CO2 previously fixed by PEPC that leaks back to the mesophyll cells. Values for leakiness can theoretically range from 0 to 1 and depend on the CO2 gradient between the two cell types, determined by the ratio of PEPC and Rubisco carboxylation rates, as well as on the conductance of bundle sheath cells (Henderson et al. 1992; von Caemmerer and Furbank 2003). A higher leakiness enables a higher discrimination by Rubisco, since it allows some 13C to be released from the bundle sheath cell. Additionally, some fractionation occurs during leakage itself (s = 1.8 ‰, Henderson et al. 1992). Overall, due to the 13C enrichment in the initial fixation step and due to the dampened Rubisco discrimination, caused by the restricted CO2 release from the bundle sheath cell, Δ13C is lower in C4 plants with values typically around 4–8 ‰ (Farquhar 1983; Henderson et al. 1992; von Caemmerer et al. 2014) as compared to 16–21 ‰ in C3 plants (Kohn 2010; O’Leary 1988). In C4 plants, it has also been described, that variation of Δ13C accompanying changes in Ci/Ca is smaller compared to C3 plants (Evans et al. 1986; Henderson et al. 1992, 1998). Depending on leakiness, the relationship between Δ13C and Ci/Ca and consequently WUE can theoretically be positive, negative or zero, with zero correlation at a leakiness of 0.37 (Farquhar et al. 1989a). Estimates of leakiness in experiments on a variety of C4 species using simultaneous measurements of on-line Δ13C and gas exchange have been reported to be lower than 0.3, leading to a positive relationship between Δ13C and WUE (Ellsworth and Cousins 2016).
In summary, compared to C3 plants a weaker correlation between Δ13C and WUE can be expected in C4 plants. If bundle sheath leakiness is relatively constant, as suggested by experimental values for sorghum (Sorghum bicolor) and Amaranthus edulis (Henderson et al. 1992, 1998; Sonawane and Cousins 2020), it should be possible to use Δ13C as a proxy trait in breeding for developing more water use efficient C4 crops.
Methods to assess carbon isotope discrimination (Δ13C)
Measurements of Δ13C values require sensitive and well-standardized methods to reduce environmental influences and temporal changes. To be applied in breeding, it is additionally important that measurements are not excessively time- and labor-intensive and can be assessed at an early developmental stage.
Short-term measurements of Δ13C, assessing the concurrent change in δ13C in the air entering and exiting a leaf cuvette, can be performed with a continuous flow isotope ratio mass spectrometer (CF-IRMS) combined with an infrared gas analyzer (Kubasek et al. 2007). These on-line measurements of Δ13C give a direct measure of the photosynthetic discrimination, allow to follow short-term changes in response to changing environmental conditions and can be used to study different components of the C4 pathway, including leakiness and mesophyll conductance (von Caemmerer et al. 2014). For the purpose of screening for WUE, these measurements are not suitable, since on-line IRMS measurements are more time-consuming and of higher cost than measuring the direct trait (Cernusak et al. 2013). An alternative to on-line measurements of Δ13C by IRMS are measurements by tunable diode laser absorption spectroscopy (TDLAS) which allow higher throughput, offer application in the field and come at lower cost. For these reasons, they have been used more frequently in Δ13C research in recent years (Ubierna et al. 2018b). The precision of TDLAS for CO2 isotopologues is reported to be 0.2 ‰ compared with ≤ 0.1 ‰ for IRMS (Cui et al. 2018). Therefore, TDLAS might be less potent to detect the small differences in ∆13C of C4 plants (Table 1). While short-term measurements of gas exchange give a direct reflection of the current photosynthetic processes, they are sensitive to environmental and developmental fluctuations (De Souza et al. 2018; Medrano et al. 2015) as well as to time and day of measurement, as gas exchange and ∆13C follow diurnal cycles (Matthews et al. 2017; Niu et al. 2003; Stangl et al. 2019).
Table 1.
Intraspecific variation of carbon isotope discrimination/composition in C4 plants
| Species | Genetic material | Carbon isotopic composition (δ13C) in ‰ | Carbon isotope discrimination (∆13C) in ‰ | Maximum genotypic difference (‰) | Tissue | References |
|---|---|---|---|---|---|---|
| Zea mays | 50 commercial inbred lines | − 11.6 to − 10.7 | 0.9 | Grain | Tieszen and Fagre (1993) | |
| 59 diverse accessions | − 12.00 to − 9.86 | 2.14 | Grain | |||
| 193 diverse accessions | − 11.5 to − 9.7 | 1.8 | Grain | |||
| 6 lines with contrasting drought tolerance, 35 hybrids, 2 drought tolerant and 2 drought sensitive inbred lines | 4.88 to 5.41 | 0.53 | Leaf | Monneveux et al. (2007) | ||
| 4.10 to 4.54 | 0.44 | Ears | ||||
| 16 hybrids, one commercial hybrid as a check | 4.98 to 5.53 | 0.55 | Leaf | Cabrera-Bosquet et al. (2009) | ||
| 3.59 to 4.01 | 0.42 | Grain | ||||
| Mean of 15 tropical inbred lines and mean of 16 of their hybrids | 5.30 to 5.64 | 0.34 | Leaf | Araus et al. (2010) | ||
| 3.82 to 4.01 | 0.19 | Grain | ||||
| 2 varieties | − 14.78 to − 13.13 | 1.65 | Leaf | Pengelly et al. (2011) | ||
| − 15.08 to − 15.02 | 0.06 | Husk | ||||
| 89 introgression lines, derived from a dent and a flint inbred line | 4.24 to 5.84 | 1.6 | Grain (field) | Gresset et al. (2014) | ||
| 4.98 to 6.55 | 1.57 | Grain (GH) | ||||
| 5.42 to 6.98 | 1.56 | Leaf (GH) | Gresset (2014) | |||
| 29 inbred lines (including 26 NAMa founders) | − 15.0 to − 13.7 | 1.3 | Leaf | Kolbe et al. (2018) | ||
| 31 inbred lines (including 26 NAM founders) | − 13.02 to − 11.61 (2015) | 1.41 | Leaf | Twohey et al. (2019) | ||
| − 13.29 to − 12.22 (2016) | 1.07 | Leaf | ||||
| Panicum coloratum | 4 varieties | − 12.74 to − 11.36 | 1.38 | Leaf | Ohsugi et al. (1988) | |
| Saccharum spp. Hybrid | 2 cultivars | 4.4 to 4.7 | 0.3 | Leaf | Meinzer et al. (1994) | |
| 4 cultivars | 3.2 to 3.9 | 0.7 | Leaf | Saliendra et al. (1996) | ||
| Sorghum bicolor Moench | 12 genotypes | 4.24 to 4.84 | 0.6 | Leaf | Hubick et al. (1990) | |
| 45 cultivars | 3.10 to 4.15 | 1.05 | Leaf | Hammer et al. (1997) | ||
| 30 lines | 2.46 to 2.89 | 0.43 | Leaf (GH) | Henderson et al. (1998) | ||
| 4 lines | 3.43 to 4.10 | 0.67 | Leaf (field) |
aNAM, nested association mapping, the NAM founder lines include the 26 most extensively researched maize lines, which represent a broad cross section of modern maize diversity (Yu et al. 2008)
GH, greenhouse
Alternatively, Δ13C can be estimated from δ13C of plant dry matter or extracted plant compounds (e.g., photosynthetic assimilates such as sugars) measured by IRMS. Differences in the δ13C of genotypes evaluated in the same experiment reflect variation in Δ13C, because it can be assumed that δ13Cair was the same for all plants. For comparison across experiments, δ13Cair has to be known or assessed to derive Δ13C (see Eq. (2)). While assessing δ13C of plant material requires destructive sampling, it has the advantage of being independent of a measurement time point. Thus, these measurements are less affected by errors due to external factors and allow high numbers of samples to be screened. Since the photosynthetic assimilates are used for plant syntheses, dry matter δ13C of, e.g., leaves or grains is assumed to be a time-integrated measure of Δ13C over the period of tissue growth (Ellsworth and Cousins 2016; Pate 2001). By integrating the diurnal, developmental and environmental fluctuations in Ci/Ca that would also affect WUEplant, dry matter δ13C has an additional advantage over on-line measurements, which can only reflect iWUE at the time point of measurement.
Differences between short-term measurements and dry matter derived estimates of Δ13C can also originate from post-photosynthetic fractionations (Henderson et al. 1992; Kubasek et al. 2007; von Caemmerer et al. 2014). Post-photosynthetic fractionations occur during metabolic reactions associated with the synthesis of different plant compounds (Hobbie and Werner 2004; Tcherkez et al. 2011) and during dark-respiration (Ghashghaie and Badeck 2014). Preferential export or incorporation of certain metabolite pools with distinct δ13C (Badeck et al. 2005, 2009; Bögelein et al. 2019) is hypothesized to then influence bulk leaf δ13C.
The sum of these additional fractionation processes can cause measurements of dry matter derived Δ13C to deviate from on-line Δ13C (Henderson et al. 1992; Kubasek et al. 2007) and can lead to weak or non-significant correlations of on-line and dry matter Δ13C over different C4 species of various C4-decarboxylation types as shown by Henderson et al. (1992) and Cousins et al. (2008). It is not established, whether post-photosynthetic fractionations significantly contribute to intraspecific variation of Δ13C in C4 plants and therefore affect the correlation of δ13C and WUE over different genotypes. The only study on this topic we are aware of was performed on diverse maize lines by Kolbe et al. (2018). Here, an RNA-sequencing approach did not reveal any indications for differences in post-photosynthetic metabolism that could be related to Δ13C differences between genotypes.
In the literature, next to leaves, grains are the most commonly sampled tissue, with absolute values for grain Δ13C being lower than for leaf Δ13C (Cabrera-Bosquet et al. 2009; Cernusak et al. 2009, Table 1). The correlation between the two measurements has been observed to be low in C4 species as well as in C3 species (Merah et al. 2001; Condon et al. 2004; Gresset 2014). On the one hand, these differences could originate from different temporal effects with leaf δ13C being more reflective of earlier vegetative growth and grain δ13C being more indicative of the conditions later in the growth period around flowering and grain filling (Condon et al. 2004; Cernusak et al. 2009). On the other hand, differences in biochemical composition or in the δ13C of sucrose exported for grain filling are likely to contribute to the disparity.
Overall, dry matter δ13C and the derived Δ13C are useful measures for screening for time-integrated WUE, if a reliable connection to WUE can be established. Dry matter δ13C is less measurement time sensitive than gas exchange measurements of iWUE and less destructive than WUEplant measurements. For the tissue to be sampled, leaves are recommendable over grains, as they resemble more closely a time-integrated measure of iWUE and allow sampling during early developmental stages.
Genetic analyses of Δ13C
Given that two of the main determinants of Δ13C, CO2 assimilation rate and stomatal conductance, are known to be complex polygenic traits in crops with C3 as well as C4 photosynthesis (Prado et al. 2018; van Bezouw et al. 2019), the genetic composition of Δ13C can be expected to be complex as well.
In C3 species, Δ13C has been shown to be determined by multiple QTL with small individual effects (Chen et al. 2011). In populations of C3 crops successfully used for QTL mapping, the intraspecific genetic variation for leaf or above-ground dry matter derived Δ13C was shown to be quantitative with maximal genotypic differences of about 1.2–2.3 ‰ in wheat (Rebetzke et al. 2008), 2.5 ‰ in soybean (Bazzer et al. 2020) and 3–4 ‰ in barley (Chen et al. 2012). Heritability was shown to be high for model plants such as Arabidopsis thaliana (0.67, Easlon et al. 2014) and crops such as wheat (0.63–0.86; Rebetzke et al. 2008). Regarding genotype by environment interactions (GxE) contrasting reports exist, but generally the genetic component seems to be much larger (Chen et al. 2011). In Arabidopsis, genes with pleiotropic effects on Δ13C, WUE and stomatal conductance have been identified (Des Marais et al. 2014; Franks et al. 2015; Masle et al. 2005; Nilson and Assmann 2010; Yang et al. 2016). Causal genes affecting Δ13C and WUE through effects on stomatal conductance were also identified in tomato (Bradford et al. 1983; Thompson et al. 2007) and potato (Antunes et al. 2012). Interactions of individual QTL for Δ13C with the genetic background have been demonstrated for several C3 crops, including soybean (Bazzer et al. 2020).
In C4 crop breeding, the use of Δ13C as an indirect selection criterion for improvement of WUE would require sufficient natural variation and a heritability comparable to C3 plants. Several studies have demonstrated that significant intraspecific variation for Δ13C exists, which might be indicative of differences in WUE and can be exploited for quantitative genetic studies to identify genomic regions controlling Δ13C. Evidence for significant intraspecific variation has been shown for maize, sorghum, sugarcane and Panicum coloratum (Table 1). For maize, several studies have explored variation in δ13C between different genotypes and results strongly depended on the investigated genetic material (Table 1). Significant genotypic differences were found mainly in sets with high genetic diversity and in material for which differences in drought tolerance and WUE were expected. For example, for δ13C in grain sampled from two diverse maize populations, a fairly large range of phenotypic values was observed (extremes differing by 2.1 ‰ and 1.8 ‰) as compared to a panel of inbred lines from a commercial breeding program with much less differentiation (0.9 ‰, Tieszen and Fagre 1993). Another example is the study of Monneveux et al. (2007), who found significant genotypic differences in leaf and ear δ13C between drought tolerant maize hybrids, drought tolerant inbred lines and susceptible inbred lines. Across drought tolerant hybrids, however, for which variation for WUE was likely reduced through previous selection for yield under drought, differences in Δ13C were small or absent. Similar results were shown in the study conducted by Cabrera-Bosquet et al. (2009), who studied maize hybrids derived from the same population with improved drought tolerance. Among a genetically diverse set of maize inbred lines frequently used in maize research (Gage et al. 2020), maximal genotypic differences of leaf δ13C were between 1.1 and 1.4 ‰ depending on the environment (Kolbe et al. 2018; Twohey et al. 2019) with a medium heritability of 0.57. High genotypic differences of up to 1.6 ‰ as well as a heritability of 0.69 have been demonstrated for grain δ13C in a maize introgression library derived from a drought tolerant dent recurrent parent and a drought susceptible flint donor parent (Avramova et al. 2019; Gresset et al. 2014). Thus, in the C4 species maize significant genotypic variation for δ13C exists, setting the stage for studies in C4 plants to investigate if δ13C could be predictive for WUE.
In recent years, several QTL studies for δ13C have been conducted in C4 plants. QTL for leaf δ13C explaining 6.5–14.5% of the genetic variance have been mapped in the C4 model grass Setaria (Ellsworth et al. 2020). In an interspecific recombinant inbred line (RIL) population of the two species Setaria viridis and Setaria italica, with a large phenotypic range for δ13C of 2.3 ‰, three QTL were identified with positive alleles contributed by both parents. Under drought-treatment with reduced variation for Ci/Ca due to stomatal closure, on the other hand, no QTL could be detected.
In maize, using an introgression library, Gresset et al. (2014) identified 22 target regions with an effect on grain δ13C distributed over all 10 chromosomes. For 12 of the 22 regions the donor parent alleles affected δ13C positively, for the remaining 10 regions negatively. Of the identified QTL, one region explained 15% of the phenotypic variance and four others more than 5%, respectively. Absolute additive effects assigned to these regions were 0.20–0.31 ‰. A recent QTL analysis of leaf δ13C in maize was based on four RIL families derived from four different inbred lines crossed to B73 as the common parent (Sorgini et al. 2020). In this study, five QTL, which explained around 7–21% of the phenotypic variance were identified. Interestingly, three of these QTL overlap with QTL identified for grain δ13C by Gresset et al. (2014). This might indicate that the detected QTL for δ13C acted independently of the genetic background and points to a connection of leaf and grain δ13C. Contrarily, none of the leaf δ13C QTL were shared between the four different RIL families (Sorgini et al. 2020). As described for Setaria by Ellsworth et al. (2020), genotypic differences in maize δ13C were found to be reduced under low precipitation in the field (Avramova et al. 2019; Twohey et al. 2019). Hence, screening for δ13C is preferably to be performed under well-watered conditions to achieve better genetic differentiation of genotypes, which has also been concluded for C3 crops (Rebetzke et al. 2008).
There are contrasting reports regarding the relevance of GxE interactions for δ13C in C4 crops. In sorghum, Henderson et al. (1998) found indications for considerable GxE interaction between different field and greenhouse experiments. In maize, Twohey et al. (2019) detected changes in the ranking of genotypes regarding δ13C between field and greenhouse only for a few genotypes and for the maize introgression library described in Gresset et al. (2014) there was no significant GxE interaction.
In summary, although genetic analyses of δ13C in C4 crops are still scarce, existing studies point to the usefulness of δ13C for indirect selection for WUE, justified by significant genetic variation and medium to high heritability. Due to its relation with stomatal conductance, screening potential for WUE is higher in well-watered compared to water limited conditions.
Correlation of δ13C and WUE in C4 plants
In addition to the requirement of significant genetic variation for both δ13C and WUE to select for more water use efficient plants, the central question to be resolved is whether a reliable correlation between δ13C and WUE exists in C4 species. In C3 crops, a positive correlation between δ13C and WUE is expected, because high Ci/Ca, corresponding to low WUE, allows for a high discrimination (Farquhar et al. 1989b). This relationship between δ13C and WUE has been shown at different levels, including correlation of on-line Δ13C and Ci/Ca (Evans et al. 1986), correlation of leaf δ13C and WUEplant (Farquhar and Richards 1984), correlation of leaf δ13C and yield under drought (Rebetzke et al. 2002) and colocalization of δ13C and WUE QTL (Adiredjo et al. 2014). In C4 plants, the correlation between δ13C and WUE could theoretically be positive or negative depending on leakiness. From the reported on-line measurements of Δ13C and Ci/Ca with values of leakiness below 0.37, a negative correlation between δ13C and WUE would be expected (Farquhar et al. 1989a; Henderson et al. 1992), which is in contrast to the positive association in C3 plants. Consistent with theory, Twohey et al. (2019) found a negative correlation between leaf δ13C and WUE as well as positive association of δ13C and transpiration over three different watering regimes in an experiment including four maize RILs. Decreases of δ13C under water deficit, when stomatal closure decreases Ci/Ca and increases WUE, have further been observed in several C4 species, including Setaria (Ellsworth et al. 2017), pearl millet (Brück et al. 2000), maize (Dercon et al. 2006), Australian C4 grasses (Ghannoum et al. 2002) and sorghum (Sonawane and Cousins 2020; Williams et al. 2001). These results indicate that changes in Ci/Ca are also reflected in δ13C of C4 species. However, these results do not demonstrate whether genotypic differences in iWUE, which are expected to be much smaller than changes in response to water deficit, are predictable from screening for δ13C.
For different genotypes of maize, Monneveux et al. (2007) demonstrated that drought tolerant hybrids and inbreds showed lower δ13C values as well as higher grain yield under drought compared to drought susceptible inbreds. They also found a negative correlation between δ13C and ear dry weight at female flowering under drought conditions for the inbred lines contrasting for drought tolerance. Within the sample of drought-tolerant hybrids, however, no correlation of δ13C and yield under drought was found, which is likely due to the low variation in δ13C and drought tolerance between the selected genotypes.
Experimental evidence of a correlation of δ13C with WUE in C4 plants over different genotypes in well-watered conditions has been reported for Setaria, maize and sorghum. The most direct indication of a connection of δ13C and WUE in C4 species comes from QTL mapping in the interspecific Setaria RIL population (Ellsworth et al. 2020). The three QTL identified to control δ13C overlapped with QTL for WUE, leaf composition, biomass and transpiration, strengthening the hypothesis that there is a genetic link between δ13C and WUE. Moreover, a negative phenotypic correlation between δ13C and WUE of -0.51 was found in the well-watered treatment. The authors concluded based on the strong allelic effect on the relationship between δ13C and WUE that δ13C might be used as a proxy for WUE in C4 species in both well-watered and water limited conditions. Evidence for a genetic link between δ13C and WUE has also been shown in maize. Building on the QTL mapping by Gresset et al. (2014), Avramova et al. (2019) showed that a QTL for δ13C on chromosome 7 also influences WUE. An introgression from the drought susceptible donor parent in this region causes a decrease in WUEplant and iWUE and an increase in grain δ13C, most likely by increasing stomatal conductance. The well-defined genetic material in this study also provided the framework to identify suitable molecular markers for selection of alleles affecting δ13C.
Further supporting evidence for a link between δ13C and WUE from experimental studies comes from weak, but significant phenotypic correlations of the two traits in 30 sorghum lines grown in the greenhouse as well as over individual plants of four lines grown in the field (Henderson et al. 1998). In this study, eight lines were selected for further investigation of Ci/Ca and leakiness by combined measurements of gas exchange and on-line Δ13C. While no significant differences were detected in leakiness, there were significant differences in Ci/Ca between the lines. In combination with the negative correlation of δ13C and WUE over the 30 lines this suggests that Ci/Ca and thus iWUE was the main driver of δ13C variation. Contrastingly, Hammer et al. (1997) found no correlation between δ13C and WUE in 45 diverse sorghum lines, which they attribute to potential variation in respiration, non-stomatal water loss or leakiness due to the high diversity of the material.
Intraspecific variation in leakiness might be responsible for the sometimes weak correlations between δ13C and WUE. Only a limited number of studies have investigated variation in leakiness across genotypes of the same species. As leakiness cannot be measured directly, it is commonly derived from combined measurements of Ci/Ca and Δ13C, using the model given in Eq. (3) (Henderson et al. 1992). The model relies on strong assumptions regarding energy production and consumption, fractionation factors and conductances of bundle sheath and mesophyll cells (Kromdijk et al. 2014). Due to the additional factors affecting dry matter δ13C that can lead to discrepancies in the relationship with short-term measurements of Ci/Ca, dry matter derived estimations are considered to be inaccurate representations of leakiness (Cousins et al. 2008). The only study we are aware of that used on-line measurements to investigate intraspecific differences in leakiness is the one by Henderson et al. (1998), in which no significant genotypic variation in leakiness of 30 sorghum lines was found.
The majority of leakiness studies focused on its responsiveness to environmental conditions to identify possible inefficiencies during the plant’s adaptation processes (Kromdijk et al. 2014). Changes of leakiness in response to environmental conditions, especially water deficit, can influence the correlation between Δ13C and WUE, as theoretically sign and magnitude of the correlation depends on leakiness (Eq. 3, Farquhar 1983). Henderson et al. (1992) demonstrated that leakiness is relatively stable over a range of temperatures, CO2 concentrations, and light intensities and in a recent study on sorghum no changes in leakiness were found in response to water deficit (Sonawane and Cousins 2020). Contrastingly, a significant response of leakiness to a high vapor pressure deficit has been observed for the C4 grass Cleistogenes squarrosa by Gong et al. (2017). The uncertainties in models used for calculating leakiness can have a large impact on its absolute values and its responses to environmental conditions (Kromdijk et al. 2014; Ubierna et al. 2018a), but the finely orchestrated coordination between PEPC and Rubisco as well as flexibility in the photosynthetic biochemistry has been proposed to constrain variations in leakiness (Bellasio and Griffiths 2014; Sun et al. 2012; Ubierna et al. 2013).
Overall, the sensitivity of detecting differences in WUE based on δ13C seems to be limited by the relatively small variation in δ13C with changes in Ci/Ca in C4 plants, but reports of significant genetic and phenotypic correlations between δ13C and WUE indicate that at least major differences in WUE should be detectable through screening for δ13C. While variation in leakiness could lower the extent to which δ13C reflects differences in Ci/Ca, it did not cancel the correlation of WUE with δ13C for the majority of the studies reviewed.
Conclusions
Using δ13C as an indirect trait to screen for WUE could facilitate the development of more water use efficient plants as one of the major challenges in breeding for climate resilience. While the relationship between δ13C and WUE in C4 crops is still less established than in C3 plants, evidence for a negative correlation of δ13C and WUE in C4 crops exists in the physiological as well as genetic context. Recent studies demonstrating the colocalization of δ13C and WUE QTL have delivered encouraging insights that it might be possible to identify plants with differential WUE through screening for δ13C. Additionally, these genetic studies greatly advance the possibilities for the identification of genes and molecular markers suitable for selection to improve WUE. Since intraspecific differences in δ13C and the correlation with WUE are less pronounced in C4 plants, it is likely that the sensitivity to detect differences in Ci/Ca is lower than in C3 plants, but pronounced differences should still be reflected in δ13C and allow for pre-screening of suitable genotypes. More research is needed for investigating the effect of intraspecific variation in leakiness and post-photosynthetic fractionations. Unraveling the factors influencing δ13C at the physiological and genetic level in a variety of agronomically important crops will elucidate the contribution of different physiological and genetic factors to the expression of δ13C and estimate the extent to which it reflects WUE. With a profound knowledge of the underlying genetic mechanisms, δ13C can assist research and breeding efforts directed at improving WUE in the context of breeding climate resilient crops.
Acknowledgements
We thank Monika Frey for critical reading and discussion of the manuscript.
Glossary
- Ci/Ca:
The ratio of intercellular to ambient CO2 partial pressure, determined by CO2 assimilation rate and stomatal conductance, assessed by gas exchange measurements of the plant leaf.
- Isotopic fractionation:
Alteration in the stable carbon isotope ratio (13C/12C), occurring as a result of physical or biochemical processes during the transport and metabolism of carbon in the plant.
- Carbon isotope discrimination (Δ13C):
The preferential assimilation of the lighter stable carbon isotope 12C over the heavier 13C during the process of photosynthesis in plants. Δ13C is calculated as the difference between the δ13C of the analyzed plant sample (δ13Cp typically plant dry matter) and δ13Cair surrounding the plant (Farquhar et al. 1982).
- Carbon isotopic composition/signature (δ13C):
The stable carbon isotopic composition of a sample, e.g. air or plant material (δ13C), expressed as the 13C/12C ratio of the sample (Rs) in reference to the 13C/12C ratio of the Pee Dee Belemnite Standard (RPDB), a fossil with an exceptionally high amount of the 13C isotope (Farquhar et al. 1982). More negative values for δ13C indicate a high discrimination against 13C. δ13C is successfully used as an indirect trait for screening for improved water use efficiency in C3 plants (Condon et al. 2004).
- Intrinsic water use efficiency (iWUE):
The ratio of CO2 assimilation rate to stomatal conductance, measured at the leaf level of the plant by means of infrared gas analyzers.
- Whole plant water use efficiency (WUEplant):
The ratio of the whole plant biomass to the total volume of water transpired by the plant.
- Water use efficiency (WUE):
The ratio of yield (grain or biomass) to water received or evapotranspired by the system (e.g. field plot Ellsworth and Cousins 2016).
- Vapor pressure deficit (VPD):
The difference between the amount of moisture in the air and the maximum air moisture at saturation.
Author contribution statement
S.E. did the main literature search and drafted the manuscript; S.B and V. A. supported S.E. in writing the manuscript and critically revised the work; C.C.S. had the idea for the article and critically revised the work; V.A. agrees to serve as the author responsible for contact and ensures communication.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported by the project “Maximizing photosynthetic efficiency in maize (FullThrottle)”, funded by the Federal Ministry of Education and Research (BMBF, Germany) within the scope of the funding initiative “Plant Breeding Research for the Bioeconomy” (funding ID: 031B0205C) and the German Research Foundation (Deutsche Forschungsgemeinschaft; DFG) through the Sonderforschungsbereich 924 (SFB924): “Molecular mechanisms regulating yield and yield stability in plants”.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adiredjo AL, Navaud O, Muños S, Langlade NB, Lamaze T, Grieu P. Genetic control of water use efficiency and leaf carbon isotope discrimination in sunflower (Helianthus annuus L) subjected to two drought scenarios. PLoS ONE. 2014;9:101218. doi: 10.1371/journal.pone.0101218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes WC, Provart NJ, Williams TC, Loureiro ME. Changes in stomatal function and water use efficiency in potato plants with altered sucrolytic activity. Plant, Cell Environ. 2012;35:747–759. doi: 10.1111/j.1365-3040.2011.02448.x. [DOI] [PubMed] [Google Scholar]
- Araus JL, Cairns JE. Field high-throughput phenotyping: the new crop breeding frontier. Trends Plant Sci. 2014;19:52–61. doi: 10.1016/j.tplants.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Araus JL, Sanchez C, Cabrera-Bosquet L. Is heterosis in maize mediated through better water use? New Phytol. 2010;187:392–406. doi: 10.1111/j.1469-8137.2010.03276.x. [DOI] [PubMed] [Google Scholar]
- Avramova V, et al. Carbon isotope composition, water use efficiency, and drought sensitivity are controlled by a common genomic segment in maize. Theor Appl Genet. 2019;132:53–63. doi: 10.1007/s00122-018-3193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badeck FW, Tcherkez G, Nogues S, Piel C, Ghashghaie J. Post-photosynthetic fractionation of stable carbon isotopes between plant organs–a widespread phenomenon. Rapid Commun Mass Spectrom. 2005;19:1381–1391. doi: 10.1002/rcm.1912. [DOI] [PubMed] [Google Scholar]
- Badeck FW, Fontaine JL, Dumas F, Ghashghaie J. Consistent patterns in leaf lamina and leaf vein carbon isotope composition across ten herbs and tree species. Rapid Commun Mass Spectrom. 2009;23:2455–2460. doi: 10.1002/rcm.4054. [DOI] [PubMed] [Google Scholar]
- Barbour MM, Warren CR, Farquhar GD, Forrester G, Brown H. Variability in mesophyll conductance between barley genotypes, and effects on transpiration efficiency and carbon isotope discrimination. Plant, Cell Environ. 2010;33:1176–1185. doi: 10.1111/j.1365-3040.2010.02138.x. [DOI] [PubMed] [Google Scholar]
- Bazzer SK, Kaler AS, Ray JD, Smith JR, Fritschi FB, Purcell LC. Identification of quantitative trait loci for carbon isotope ratio (δ13C) in a recombinant inbred population of soybean. Theor Appl Genet. 2020;133:2141–2155. doi: 10.1007/s00122-020-03586-0. [DOI] [PubMed] [Google Scholar]
- Bellasio C, Griffiths H. Acclimation to low light by C4 maize: implications for bundle sheath leakiness. Plant, Cell Environ. 2014;37:1046–1058. doi: 10.1111/pce.12194. [DOI] [PubMed] [Google Scholar]
- Blankenagel S, Yang Z, Avramova V, Schön C-C, Grill E. Generating plants with improved water use efficiency. Agronomy. 2018 doi: 10.3390/agronomy8090194. [DOI] [Google Scholar]
- Bögelein R, Lehmann MM, Thomas FM. Differences in carbon isotope leaf-to-phloem fractionation and mixing patterns along a vertical gradient in mature European beech and Douglas fir. New Phytol. 2019;222:1803–1815. doi: 10.1111/nph.15735. [DOI] [PubMed] [Google Scholar]
- Bradford KJ, Sharkey TD, Farquhar GD. Gas exchange, stomatal behavior, and δ13C values of the flacca tomato mutant in relation to abscisic acid. Plant Physiol. 1983;72:245–250. doi: 10.1104/pp.72.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brück H, Payne W, Sattelmacher B. Effects of phosphorus and water supply on yield, transpirational water-use efficiency, and carbon isotope discrimination of pearl millet. Crop Sci. 2000;40:120–125. doi: 10.2135/cropsci2000.401120x. [DOI] [Google Scholar]
- Cabrera-Bosquet L, Sanchez C, Araus JL. How yield relates to ash content, Δ13C and Δ18O in maize grown under different water regimes. Ann Bot. 2009;104:1207–1216. doi: 10.1093/aob/mcp229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernusak LA, et al. Why are non-photosynthetic tissues generally 13C enriched compared with leaves in C3 plants? Review and synthesis of current hypotheses. Funct Plant Biol. 2009;36:199–213. doi: 10.1071/FP08216. [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Ubierna N, Winter K, Holtum JA, Marshall JD, Farquhar GD. Environmental and physiological determinants of carbon isotope discrimination in terrestrial plants. New Phytol. 2013;200:950–965. doi: 10.1111/nph.12423. [DOI] [PubMed] [Google Scholar]
- Chen J, Chang SX, Anyia AO. Gene discovery in cereals through quantitative trait loci and expression analysis in water-use efficiency measured by carbon isotope discrimination. Plant, Cell Environ. 2011;34:2009–2023. doi: 10.1111/j.1365-3040.2011.02397.x. [DOI] [PubMed] [Google Scholar]
- Chen J, Chang SX, Anyia AO. Quantitative trait loci for water-use efficiency in barley (Hordeum vulgare L.) measured by carbon isotope discrimination under rain-fed conditions on the Canadian Prairies. Theor Appl Genet. 2012;125:71–90. doi: 10.1007/s00122-012-1817-7. [DOI] [PubMed] [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD. Breeding for high water-use efficiency. J Exp Bot. 2004;55:2447–2460. doi: 10.1093/jxb/erh277. [DOI] [PubMed] [Google Scholar]
- Cousins AB, Badger MR, von Caemmerer S. C4 photosynthetic isotope exchange in NAD-ME- and NADP-ME-type grasses. J Exp Bot. 2008;59:1695–1703. doi: 10.1093/jxb/ern001. [DOI] [PubMed] [Google Scholar]
- Cui X, et al. Environmental application of high sensitive gas sensors with tunable diode laser absorption spectroscopy. Green Electron. 2018 doi: 10.5772/intechopen.72948. [DOI] [Google Scholar]
- De Souza AP, Grandis A, Arenque-Musa BC, Buckeridge MS. Diurnal variation in gas exchange and nonstructural carbohydrates throughout sugarcane development. Funct Plant Biol. 2018;45:865–876. doi: 10.1071/FP17268. [DOI] [PubMed] [Google Scholar]
- Dercon G, Clymans E, Diels J, Merckx R, Deckers J. Differential 13C isotopic discrimination in maize at varying water stress and at low to high nitrogen availability. Plant Soil. 2006;282:313–326. doi: 10.1007/s11104-006-0001-8. [DOI] [Google Scholar]
- Des Marais DL, Auchincloss LC, Sukamtoh E, McKay JK, Logan T, Richards JH, Juenger TE. Variation in MPK12 affects water use efficiency in Arabidopsis and reveals a pleiotropic link between guard cell size and ABA response. Proc Natl Acad Sci U S A. 2014;111:2836–2841. doi: 10.1073/pnas.1321429111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easlon HM, Nemali KS, Richards JH, Hanson DT, Juenger TE, McKay JK. The physiological basis for genetic variation in water use efficiency and carbon isotope composition in Arabidopsis thaliana. Photosynth Res. 2014;119:119–129. doi: 10.1007/s11120-013-9891-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth PZ, Cousins AB. Carbon isotopes and water use efficiency in C4 plants. Curr Opin Plant Biol. 2016;31:155–161. doi: 10.1016/j.pbi.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Ellsworth PZ, Ellsworth PV, Cousins AB. Relationship of leaf oxygen and carbon isotopic composition with transpiration efficiency in the C4 grasses Setaria viridis and Setaria italica. J Exp Bot. 2017;68:3513–3528. doi: 10.1093/jxb/erx185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth PZ, Feldman MJ, Baxter I, Cousins AB. A genetic link between leaf carbon isotope composition and whole-plant water use efficiency in the C4 grass Setaria. Plant J. 2020;102:1234–1248. doi: 10.1111/tpj.14696. [DOI] [PubMed] [Google Scholar]
- Evans JR, Sharkey TD, Berry JA, Farquhar GD. Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Funct Plant Biol. 1986;13:281–292. doi: 10.1071/PP9860281. [DOI] [Google Scholar]
- Farquhar GD. On the nature of carbon isotope discrimination in C4 species. Funct Plant Biol. 1983;10:205–226. doi: 10.1071/PP9830205. [DOI] [Google Scholar]
- Farquhar GD, Cernusak LA. Ternary effects on the gas exchange of isotopologues of carbon dioxide. Plant, Cell Environ. 2012;35:1221–1231. doi: 10.1111/j.1365-3040.2012.02484.x. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Richards RA. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Funct Plant Biol. 1984;11:539–552. doi: 10.1071/PP9840539. [DOI] [Google Scholar]
- Farquhar GD, O’Leary MH, Berry JA. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Funct Plant Biol. 1982;9:121–137. doi: 10.1071/PP9820121. [DOI] [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annual Rev Plant Biol. 1989;40:503–537. doi: 10.1146/annurev.pp.40.060189.002443. [DOI] [Google Scholar]
- Farquhar GD, Hubick KT, Condon AG, Richards RA. Carbon isotope fractionation and plant water-use efficiency. Stable isotopes in ecological research. New York: Springer; 1989. pp. 21–40. [Google Scholar]
- Franks PJ, Doheny-Adams T, Britton-Harper ZJ, Gray JE. Increasing water-use efficiency directly through genetic manipulation of stomatal density. New Phytol. 2015;207:188–195. doi: 10.1111/nph.13347. [DOI] [PubMed] [Google Scholar]
- Gage JL, Monier B, Giri A, Buckler ES. Ten years of the maize nested association mapping population: impact, limitations, and future directions. Plant Cell. 2020 doi: 10.1105/tpc.19.00951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetika G, van Oosterom EJ, George-Jaeggli B, Mortlock MY, Deifel KS, McLean G, Hammer GL. Genotypic variation in whole-plant transpiration efficiency in sorghum only partly aligns with variation in stomatal conductance. Funct Plant Biol. 2019;46:1072–1089. doi: 10.1071/FP18177. [DOI] [PubMed] [Google Scholar]
- Ghannoum O, von Caemmerer S, Conroy JP. The effect of drought on plant water use efficiency of nine NAD-ME and nine NADP-ME Australian C4 grasses. Funct Plant Biol. 2002;29:1337–1348. doi: 10.1071/FP02056. [DOI] [PubMed] [Google Scholar]
- Ghashghaie J, Badeck FW. Opposite carbon isotope discrimination during dark respiration in leaves versus roots—a review. New Phytol. 2014;201:751–769. doi: 10.1111/nph.12563. [DOI] [PubMed] [Google Scholar]
- Gong XY, Schäufele R, Schnyder H. Bundle-sheath leakiness and intrinsic water use efficiency of a perennial C4 grass are increased at high vapour pressure deficit during growth. J Exp Bot. 2017;68:321–333. doi: 10.1093/jxb/erw417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graven H, et al. Compiled records of carbon isotopes in atmospheric CO2 for historical simulations in CMIP6. Geosci Model Develop. 2017;10:4405–4417. doi: 10.5194/gmd-10-4405-2017. [DOI] [Google Scholar]
- Gresset S (2014) Genetische Analyse der stabilen Kohlenstoffisotopendiskriminierung in einer Mais (Zea mays L.) Introgressionsbibliothek. Technische Universität München
- Gresset S, Westermeier P, Rademacher S, Ouzunova M, Presterl T, Westhoff P, Schön CC. Stable carbon isotope discrimination is under genetic control in the C4 species maize with several genomic regions influencing trait expression. Plant Physiol. 2014;164:131–143. doi: 10.1104/pp.113.224816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer GL, Farquhar GD, Broad IJ. On the extent of genetic variation for transpiration efficiency in sorghum. Aust J Agric Res. 1997;48:649–656. doi: 10.1071/A96111. [DOI] [Google Scholar]
- Hatfield JL, Dold C. Water-use efficiency: advances and challenges in a changing climate. Front Plant Sci. 2019;10:103. doi: 10.3389/fpls.2019.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson SA, von Caemmerer S, Farquhar GD. Short-term measurements of carbon isotope discrimination in several C4 species. Funct Plant Biol. 1992;19:263–285. doi: 10.1071/PP9920263. [DOI] [Google Scholar]
- Henderson S, Von Caemmerer S, Farquhar G, Wade L, Hammer G. Correlation between carbon isotope discrimination and transpiration efficiency in lines of the C4 species Sorghum bicolor in the glasshouse and the field. Funct Plant Biol. 1998;25:111–123. doi: 10.1071/PP95033. [DOI] [Google Scholar]
- Hobbie EA, Werner RA. Intramolecular, compound-specific, and bulk carbon isotope patterns in C3 and C4 plants: a review and synthesis. New Phytol. 2004;161:371–385. doi: 10.1111/j.1469-8137.2004.00970.x. [DOI] [PubMed] [Google Scholar]
- Hubick K, Farquhar G. Carbon isotope discrimination and the ratio of carbon gained to water lost in barley cultivars. Plant, Cell Environ. 1989;12:795–804. doi: 10.1111/j.1365-3040.1989.tb01641.x. [DOI] [Google Scholar]
- Hubick KT, Farquhar GD, Shorter R. Correlation between water-use efficiency and carbon isotope discrimination in diverse peanut (Arachis) germplasm. Funct Plant Biol. 1986;13:803–816. doi: 10.1071/PP9860803. [DOI] [Google Scholar]
- Hubick KT, Hammer GL, Farquhar GD, Wade LJ, von Caemmerer S, Henderson SA. Carbon isotope discrimination varies genetically in C4 species. Plant Physiol. 1990;92:534–537. doi: 10.1104/pp.92.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impa SM, Nadaradjan S, Boominathan P, Shashidhar G, Bindumadhava H, Sheshshayee MS. Carbon isotope discrimination accurately reflects variability in WUE measured at a whole plant level in rice. Crop Sci. 2005;45:2517–2522. doi: 10.2135/cropsci2005.0119. [DOI] [Google Scholar]
- Jackson P, Basnayake J, Inman-Bamber G, Lakshmanan P, Natarajan S, Stokes C. Genetic variation in transpiration efficiency and relationships between whole plant and leaf gas exchange measurements in Saccharum spp. and related germplasm. J Exp Bot. 2016;67:861–871. doi: 10.1093/jxb/erv505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn MJ. Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo)ecology and (paleo)climate. Proc Natl Acad Sci U S A. 2010;107:19691–19695. doi: 10.1073/pnas.1004933107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbe AR, Studer AJ, Cousins AB. Biochemical and transcriptomic analysis of maize diversity to elucidate drivers of leaf carbon isotope composition. Funct Plant Biol. 2018;45:489–500. doi: 10.1071/FP17265. [DOI] [PubMed] [Google Scholar]
- Kromdijk J, Ubierna N, Cousins AB, Griffiths H. Bundle-sheath leakiness in C4 photosynthesis: a careful balancing act between CO2 concentration and assimilation. J Exp Bot. 2014;65:3443–3457. doi: 10.1093/jxb/eru157. [DOI] [PubMed] [Google Scholar]
- Kubasek J, Setlik J, Dwyer S, Santrucek J. Light and growth temperature alter carbon isotope discrimination and estimated bundle sheath leakiness in C4 grasses and dicots. Photosynth Res. 2007;91:47–58. doi: 10.1007/s11120-007-9136-6. [DOI] [PubMed] [Google Scholar]
- Leakey ADB, Ferguson JN, Pignon CP, Wu A, Jin Z, Hammer GL, Lobell DB. Water use efficiency as a constraint and target for improving the resilience and productivity of C3 and C4 crops. Annu Rev Plant Biol. 2019;70:781–808. doi: 10.1146/annurev-arplant-042817-040305. [DOI] [PubMed] [Google Scholar]
- Martin B, Tauer CG, Lin RK. Carbon isotope discrimination as a tool to improve water-use efficiency in tomato. Crop Sci. 1999;39:1775–1783. doi: 10.2135/cropsci1999.3961775x. [DOI] [Google Scholar]
- Masle J, Gilmore SR, Farquhar GD. The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature. 2005;436:866–870. doi: 10.1038/nature03835. [DOI] [PubMed] [Google Scholar]
- Matthews JSA, Vialet-Chabrand SRM, Lawson T. Diurnal variation in gas exchange: the balance between carbon fixation and water loss. Plant Physiol. 2017;174:614–623. doi: 10.1104/pp.17.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano H, et al. From leaf to whole-plant water use efficiency (WUE) in complex canopies: limitations of leaf WUE as a selection target. Crop J. 2015;3:220–228. doi: 10.1016/j.cj.2015.04.002. [DOI] [Google Scholar]
- Meinzer FC, Plaut Z, Saliendra NZ. Carbon isotope discrimination, gas exchange, and growth of sugarcane cultivars under salinity. Plant Physiol. 1994;104:521–526. doi: 10.1104/pp.104.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merah O, Deléens E, Souyris I, Nachit M, Monneveux P. Stability of carbon isotope discrimination and grain yield in durum wheat. Crop Sci. 2001;41:677–681. doi: 10.2135/cropsci2001.413677x. [DOI] [PubMed] [Google Scholar]
- Monneveux P, Sheshshayee MS, Akhter J, Ribaut J-M. Using carbon isotope discrimination to select maize (Zea mays L.) inbred lines and hybrids for drought tolerance. Plant Sci. 2007;173:390–396. doi: 10.1016/j.plantsci.2007.06.003. [DOI] [Google Scholar]
- Nilson SE, Assmann SM. The alpha-subunit of the Arabidopsis heterotrimeric G protein, GPA1, is a regulator of transpiration efficiency. Plant Physiol. 2010;152:2067–2077. doi: 10.1104/pp.109.148262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu S, Jiang G, Li Y, Gao L, Liu M. Diurnal gas exchange and superior resources use efficiency of typical C4 species in Hunshandak Sandland, China. Photosynthetica. 2003;41:221–226. doi: 10.1023/B:PHOT.0000011954.32698.ea. [DOI] [Google Scholar]
- Ohsugi R, Samejima M, Chonan N, Murata T. δ13C values and the occurrence of suberized lamellae in some panicum species. Ann Bot. 1988;62:53–59. doi: 10.1093/oxfordjournals.aob.a087635. [DOI] [Google Scholar]
- O’Leary MH. Carbon isotopes in photosynthesis. Bioscience. 1988;38:328–336. doi: 10.2307/1310735. [DOI] [Google Scholar]
- Pate JS. Carbon isotope discrimination and plant water-use efficiency. Stable isotope techniques in the study of biological processes and functioning of ecosystems. Berlin: Springer; 2001. pp. 19–36. [Google Scholar]
- Pengelly JJ, et al. Functional analysis of corn husk photosynthesis. Plant Physiol. 2011;156:503–513. doi: 10.1104/pp.111.176495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado SA, Cabrera-Bosquet L, Grau A, Coupel-Ledru A, Millet EJ, Welcker C, Tardieu F. Phenomics allows identification of genomic regions affecting maize stomatal conductance with conditional effects of water deficit and evaporative demand. Plant, Cell Environ. 2018;41:314–326. doi: 10.1111/pce.13083. [DOI] [PubMed] [Google Scholar]
- Rebetzke GJ, Condon AG, Richards R, Farquhar G. Selection for reduced carbon isotope discrimination increases aerial biomass and grain yield of rainfed bread wheat. Crop Sci. 2002;42:739–745. doi: 10.2135/cropsci2002.0739. [DOI] [Google Scholar]
- Rebetzke GJ, Condon AG, Farquhar GD, Appels R, Richards RA. Quantitative trait loci for carbon isotope discrimination are repeatable across environments and wheat mapping populations. Theor Appl Genet. 2008;118:123–137. doi: 10.1007/s00122-008-0882-4. [DOI] [PubMed] [Google Scholar]
- Ryan AC, Dodd IC, Rothwell SA, Jones R, Tardieu F, Draye X, Davies WJ. Gravimetric phenotyping of whole plant transpiration responses to atmospheric vapour pressure deficit identifies genotypic variation in water use efficiency. Plant Sci. 2016;251:101–109. doi: 10.1016/j.plantsci.2016.05.018. [DOI] [PubMed] [Google Scholar]
- Saliendra NZ, Meinzer FC, Perry M, Thom M. Associations between partitioning of carboxylase activity and bundle sheath leakiness to CO2, carbon isotope discrimination, photosynthesis, and growth in sugarcane. J Exp Bot. 1996;47:907–914. doi: 10.1093/jxb/47.7.907. [DOI] [Google Scholar]
- Saranga Y, Flash I, Yakir D. Variation in water-use efficiency and its relation to carbon isotope ratio in cotton. Crop Sci. 1998;38:782–787. doi: 10.2135/cropsci1998.0011183X003800030027x. [DOI] [Google Scholar]
- Sinclair TR. Is transpiration efficiency a viable plant trait in breeding for crop improvement? Funct Plant Biol. 2012;39:359–365. doi: 10.1071/FP11198. [DOI] [PubMed] [Google Scholar]
- Sonawane BV, Cousins AB. Mesophyll CO2 conductance and leakiness are not responsive to short- and long-term soil water limitations in the C4 plant Sorghum bicolor. Plant J. 2020 doi: 10.1111/tpj.14849. [DOI] [PubMed] [Google Scholar]
- Sorgini CA, Roberts LM, Cousins AB, Baxter I, Studer AJ (2020) The genetic architecture of leaf stable carbon isotope composition in Zea mays and the effect of transpiration efficiency on elemental accumulation. 10.1101/2020.03.12.989509 [DOI] [PMC free article] [PubMed]
- Stangl ZR, Tarvainen L, Wallin G, Ubierna N, Rantfors M, Marshall JD. Diurnal variation in mesophyll conductance and its influence on modelled water-use efficiency in a mature boreal Pinus sylvestris stand. Photosynth Res. 2019;141:53–63. doi: 10.1007/s11120-019-00645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Ubierna N, Ma JY, Cousins AB. The influence of light quality on C4 photosynthesis under steady-state conditions in Zea mays and Miscanthus x giganteus: changes in rates of photosynthesis but not the efficiency of the CO2 concentrating mechanism. Plant, Cell Environ. 2012;35:982–993. doi: 10.1111/j.1365-3040.2011.02466.x. [DOI] [PubMed] [Google Scholar]
- Tcherkez G, Mahe A, Hodges M. 12C/13C fractionations in plant primary metabolism. Trends Plant Sci. 2011;16:499–506. doi: 10.1016/j.tplants.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Thompson AJ, et al. Overproduction of abscisic acid in tomato increases transpiration efficiency and root hydraulic conductivity and influences leaf expansion. Plant Physiol. 2007;143:1905–1917. doi: 10.1104/pp.106.093559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieszen LL, Fagre T. Carbon isotopic variability in modern and archaeological maize. J Archaeol Sci. 1993;20:25. doi: 10.1006/jasc.1993.1002. [DOI] [Google Scholar]
- Twohey RJ, III, Roberts LM, Studer AJ. Leaf stable carbon isotope composition reflects transpiration efficiency in Zea mays. Plant J. 2019;97:475–484. doi: 10.1111/tpj.14135. [DOI] [PubMed] [Google Scholar]
- Ubierna N, Sun W, Kramer DM, Cousins AB. The efficiency of C4 photosynthesis under low light conditions in Zea mays, Miscanthus x giganteus and Flaveria bidentis. Plant, Cell Environ. 2013;36:365–381. doi: 10.1111/j.1365-3040.2012.02579.x. [DOI] [PubMed] [Google Scholar]
- Ubierna N, Gandin A, Cousins AB. The response of mesophyll conductance to short-term variation in CO2 in the C4 plants Setaria viridis and Zea mays. J Exp Bot. 2018;69:1159–1170. doi: 10.1093/jxb/erx464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubierna N, Holloway-Phillips M-M, Farquhar GD (2018b) Using stable carbon isotopes to study C3 and C4 photosynthesis: models and calculations. In: Photosynthesis. Springer, pp 155–196 [DOI] [PubMed]
- van Bezouw R, Keurentjes JJB, Harbinson J, Aarts MGM. Converging phenomics and genomics to study natural variation in plant photosynthetic efficiency. Plant J. 2019;97:112–133. doi: 10.1111/tpj.14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S, Furbank RT. The C4 pathway: an efficient CO2 pump. Photosynth Res. 2003;77:191. doi: 10.1023/A:1025830019591. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Ghannoum O, Pengelly JJ, Cousins AB. Carbon isotope discrimination as a tool to explore C4 photosynthesis. J Exp Bot. 2014;65:3459–3470. doi: 10.1093/jxb/eru127. [DOI] [PubMed] [Google Scholar]
- Vos J, Groenwold J. Genetic differences in water-use efficiency, stomatal conductance and carbon isotope fractionation in potato. Potato Res. 1989;32:113–121. doi: 10.1007/BF02358219. [DOI] [Google Scholar]
- Way DA, Katul GG, Manzoni S, Vico G. Increasing water use efficiency along the C3 to C4 evolutionary pathway: a stomatal optimization perspective. J Exp Bot. 2014;65:3683–3693. doi: 10.1093/jxb/eru205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D, et al. Carbon isotope discrimination by Sorghum bicolor under CO2 enrichment and drought. New Phytol. 2001;150:285–293. doi: 10.1046/j.1469-8137.2001.00093.x. [DOI] [Google Scholar]
- Xin Z, Aiken R, Burke J. Genetic diversity of transpiration efficiency in sorghum. Field Crops Res. 2009;111:74–80. doi: 10.1016/j.fcr.2008.10.010. [DOI] [Google Scholar]
- Yang Z, Liu J, Tischer SV, Christmann A, Windisch W, Schnyder H, Grill E. Leveraging abscisic acid receptors for efficient water use in Arabidopsis. Proc Natl Acad Sci U S A. 2016;113:6791–6796. doi: 10.1073/pnas.1601954113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Holland JB, McMullen MD, Buckler ES. Genetic design and statistical power of nested association mapping in maize. Genetics. 2008;178:539–551. doi: 10.1534/genetics.107.074245. [DOI] [PMC free article] [PubMed] [Google Scholar]