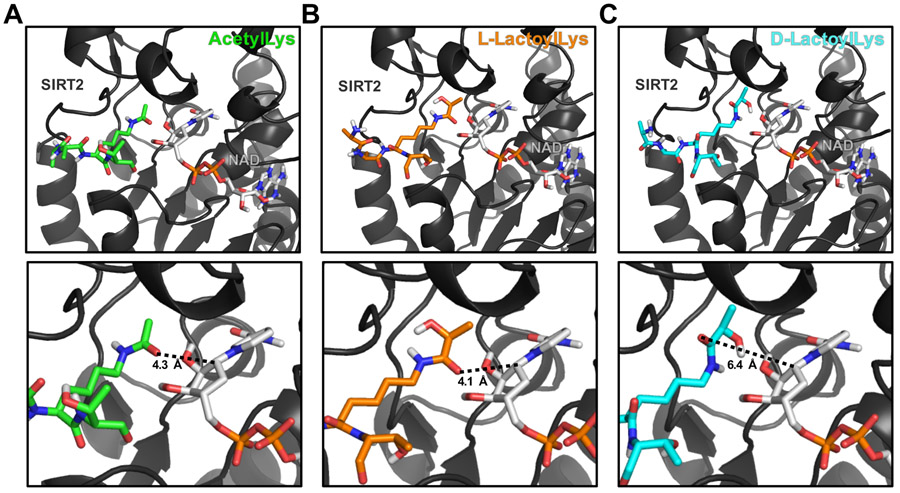
Figure 3.
Molecular modeling provides insights into enantiomeric specificity of lactoylLys modifications. Carba-NAD+ is shown on the right side of each panel. (A) Molecular modeling of an acetylLys peptide (green) docked to SIRT2 with carba-NAD+ in a catalytically competent conformation. Predicted distance from acyl carbonyl group to C1’ of the nicotinamide ribose ring is 4.3 Å. (B) L-lactoylLys peptide (orange) docked to SIRT2 with carba-NAD+ in a catalytically competent conformation. Predicted distance from acyl carbonyl group to C1’ of the nicotinamide ribose ring is 4.1 Å. (C) D-lactoylLys peptide (cyan) docked to SIRT2 in a catalytically incompetent conformation. Predicted distance from acyl carbonyl group to C1’ of the nicotinamide ribose ring is 6.4 Å. SIRT2 PDB: 5G4C; Peptide sequence: AA-K*-T.