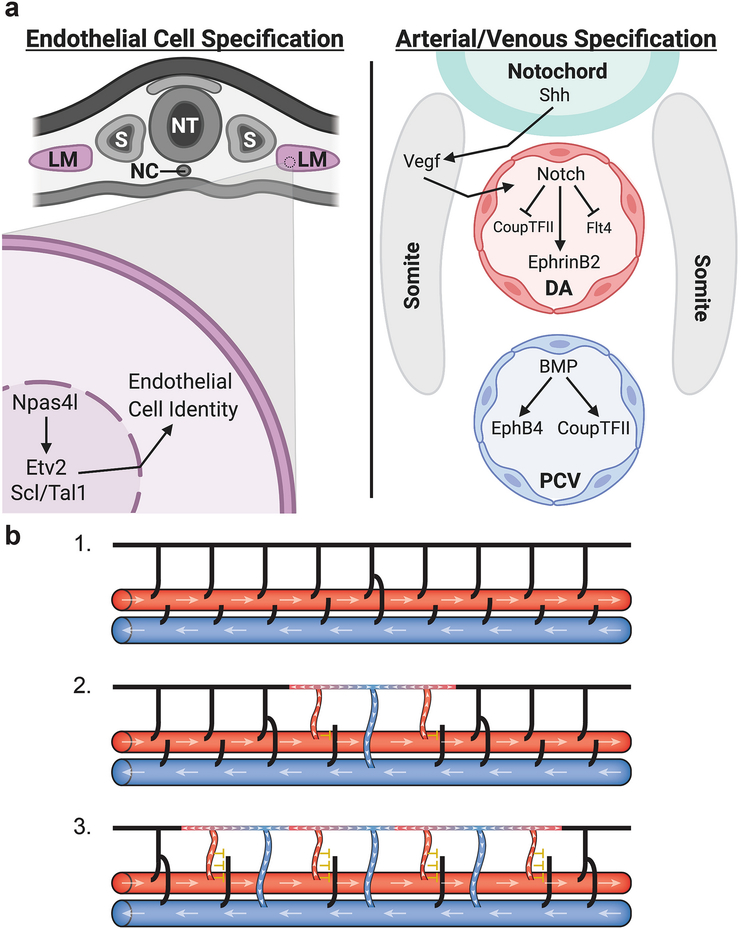
Fig 3:
Diagrams depicting the specification of arterial and venous identity through molecular mechanisms and hemodynamic forces. (a) Endothelial cells are first specified in the lateral mesoderm (LM); in zebrafish the master regulator Npas4l activates Etv2 and Scl/Tal1 to promote endothelial identity. Once specified, endothelial cells migrate out of the lateral mesoderm to form the major axial vessels - the dorsal aorta (DA) and the posterior cardinal vein (PCV). Sonic hedgehog (Shh) signaling from the notochord activates Vegf signaling in the somites which in turn activates Notch signaling in the DA leading to the expression of arterial genes and the inhibition of venous identity. In the PCV, BMP signaling promotes the expression of venous identity genes. (b) Hemodynamic forces help drive the formation of venous intersegmental vessels in the zebrafish trunk. Initially all intersegmental vessels lack flow (black) and are connected to the DA (large red horizontal vessel) (1). Once a secondary sprout from the PCV (large blue horizontal vessel) connects to a primary sprout a circuit is created allowing blood flow (white arrowheads) to move up through adjacent primary sprouts (red) and return through the venous sprout (blue) (2). Blood flow through these primary sprouts prevents secondary sprouts from connecting (yellow inhibitory T) solidifying their arterial identity. Differences in flow then permit the next set of secondary sprouts to connect (3) leading to a largely alternating pattern of arteries and veins throughout the fish trunk and a close to 50:50 artery to vein ratio. Adapted from Isogai et al. 2003 [9]. DA, dorsal aorta; LM, lateral mesoderm, NC, notochord; NT, neural tube; PCV, posterior cardinal vein; S, somite. Figure (a) was created using biorender.com.