Abstract
Background:
There is great degree of inter-observer variability in the visual angiographic assessment of LMCD. Fractional flow reserve and intravascular ultrasound (IVUS) are often used in this setting. The use of iFR for evaluation of LMCD has not been well studied. The aim of this study is to evaluate the use of instantaneous wave-free ratio (iFR) in the assessment of angiographically intermediate left main (LMCD).
Methods:
This is an international multicenter retrospective observational study of patients who underwent both iFR and IVUS evaluation for angiographically intermediate LMCD. An independent core laboratory performed blinded off-line analysis of all IVUS data. A minimum lumen area (MLA) of 6 mm2 was used as the cutoff for significant disease.
Results:
125 patients (mean age 68.4 ± 9.5 years, 84.8% male) were included in this analysis. Receiver operating curve (ROC) analysis showed that an iFR of ≤0.89 identified MLA <6 mm2 with an area under the curve of 0.77 (77% sensitivity, 66% specificity; P <0.0001). Among the 69 patients without ostial left anterior descending artery or left circumflex artery disease, ROC analysis showed that an iFR of ≤0.89 identified MLA <6 mm2 with an area under the curve of 0.84 (70% sensitivity, 84% specificity; P <0.0001). The correlation was not significantly different when the body surface area (BSA) was considered.
Conclusions:
In this study, in patients with intermediate LMCD, iFR of ≤0.89 correlates with IVUS MLA <6 mm2 regardless of BSA. The current study supports the use of iFR for the evaluation of intermediate LMCD.
Keywords: Left main coronary artery disease, iFR, Intravascular ultrasound, Imaging, Catheter-Based Coronary and Valvular Interventions
Introduction
There is a great degree of inter-observer variability in the visual angiographic assessment of left main coronary disease (LMCD), more so than any other coronary segment 1. This has led to the widespread use of multiple adjunctive modalities including anatomical evaluation with intravascular ultrasound (IVUS) and optical coherence tomography, as well as physiologic evaluation with fractional flow reserve (FFR). Both IVUS and FFR have been validated, and are frequently used as adjunctive diagnostic modalities in the evaluation of LM disease. An FFR value of 0.80 and a minimum lumen area (MLA) on IVUS of 6 mm2 are the separately accepted cutoff points above which LMCD intervention can be safely deferred with favorable long-term outcomes 2–9. In the largest left main trial to date (EXCEL), in patients with intermediate LMCD, IVUS with an MLA ≤ 6mm2 was strongly recommended as the preferred diagnostic modality, over physiology, to qualify for randomization 10. Ostial and mid LMCD assessment with IVUS can be prone to error depending on co-axiality of the guide with the vessel. Small variations in angle can lead to overestimation of MLA with distortion of the lumen shape on IVUS, therefore great care must be taken with technique and co-axiality; this may not be possible depending on the angle of left main take off. Additionally, IVUS is an anatomic, non-physiologic assessment; the accepted cutoff used can theoretically be affected by the body surface area (BSA) of the patient as evidenced by variations in described optimal cutoff MLA values, particularly in different patient cohorts such as Asian populations 11.
In recent years, instantaneous wave-free ratio (iFR), when compared to FFR, has proven to be safe for deferral of revascularization of intermediate lesions using a cutoff of 0.89, with improved median procedural time, and decrease adverse procedural effects secondary to adenosine administration 12, 13. This has led to increasing use of iFR. However, the use of iFR in LMCD had not been well established or validated, as patients with LMCD were mostly excluded from both the iFR-SWEDEHEART and the DEFINE-FLAIR studies. We have recently reported on the safety of iFR guided revascularization in 314 patients. The DEFINE LM registry, evaluated the prognostic significance of an iFR cutoff of 0.89 in the LM; clinical outcomes were similar in patients with iFR >0.89 who were not revascularized, compared to patients with iFR ≤0.89 who were revascularized 14. The study concluded that deferral of revascularization of intermediate LM stenosis using iFR cutoff 0.89 appears to be safe. In this study, we aim to evaluate the correlation between iFR and IVUS MLA to help further guide the clinical care of patients with LM disease.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Patient Population
This international multicenter retrospective cohort study was approved by the Mayo Foundation Institutional Review Board (IRB# 19-003352) and local ethics committee at each participating center, and was conducted in accordance with the guideline of the Declaration of Helsinki. Consecutive patients were enrolled from January 1, 2013 to June 30, 2019 at 10 centers in the USA, Japan, and Europe (Spain and United Kingdom). Patients included had stable angina and angiographically intermediate LMCD (40% to 70% stenosis on visual angiographic assessment) noted during clinically indicated coronary angiography and were further evaluated with IVUS and iFR. Patients were identified via query of electronic medical records for left main coronary artery disease, and were excluded from analysis if either IVUS or iFR were not attempted or were unable to be obtained. If the bifurcation lesion involved in LM and the ostial LAD or LCX, the lesion was considered to be LMCD. Patients with downstream stenoses in the LAD or LCX were also included. All patients provided written informed consent.
Data Collection
Baseline demographics and comorbidities were collected including age, sex, race, height, weight, significant family history of coronary disease, as well as medical conditions including hypertension, dyslipidemia, diabetes, chronic kidney disease, tobacco use, prior myocardial infarction, and cardiomyopathy. Angiographic findings such as location of LMCD (ostium, mid, or distal bifurcation), Medina classification for bifurcation lesions, SYNTAX score, and quantitative coronary analysis (QCA) measurements were recorded, as well as iFR values and IVUS parameters including MLA, lumen area stenosis, and atheroma burden. QCA analysis done by the individual sites. All IVUS images (0.1 mm interval) were independently reviewed off-line by a core laboratory at the Mayo Clinic using standard techniques with automated software algorithms (QIvus 3.1, MEDIS medical imaging systems Inc., NC, USA) per the American College of Cardiology clinical expert consensus document on standards for acquisition, measurement, and reporting of IVUS studies 15. MLA was measured at which the lumen was the smallest within the left main coronary artery above the bifurcation. The interobserver intraclass correlation coefficients for MLA in randomly selected 20 patients was 0.97. Reference lumen area was measured at which the lumen was the largest within 10 mm of MLA segment with no intervening branches. Percent area stenosis was calculated as (reference lumen area – MLA)/reference lumen area × 100 (%). IVUS was obtained using a variety of equipment, including Boston Opticross (n = 21), Philips Volcano (n=54), and Terumo (n=50). IVUS pullback was performed manually (n=22) or automatically at a preset speed of 0.5 mm/s (n=39) or 1 mm/s (n=64).
Standard protocol was followed for iFR measurements with administration of intracoronary nitroglycerine prior to measurement, use of a Philips Verrata wire (San Diego, CA, USA), and pullback confirmation for lack of drift in pressure measurements. iFR was measured at the distal point of the left main segment either in LAD or in LCX at the non-diseased segment. iFR was measured in LAD in 103 patients (82%), in LCX in 11 patients (9%), and in both LAD and LCX in 10 patients (9%). The higher iFR value was chosen as the representative iFR value for the patient when iFR was recorded in both LAD and LCX (6 patients had ostial lesions in both LAD and LCX with both iFR values ≤0.89; the remaining 4 patients had ostial LAD diseases and higher iFR values measured in LCX was chosen). FFR was simultaneously measured in 75 patients (60%) with standard protocol.
Statistical Analysis
Continuous variables are presented as mean ± standard deviation, and those with a skewed distribution are presented as the median with interquartile range (IQR). Categorical variables are presented as counts and percentages. For between-group comparisons, unpaired t-test was used for normally distributed continuous variables, Mann-Whitney U test for non-normally distributed variables, and chi-square or Fisher exact tests for categorical variables. Receiver-operating curve analysis was performed to assess the discriminative power of iFR value for MLA of <6 mm2 and of MLA/body surface area (BSA) for iFR value of ≤0.89 to define the sensitivity and specificity. The optimal cutoff values were identified as the value for which the sum of the sensitivity and specificity was the greatest. The correlations between two variables were assessed using the Spearman rank-sum test. For all tests, a two-tailed P value <0.05 was considered to be statistically significant. All statistical analyses were performed using JMP Pro software (SAS Institute, Inc., Cary, NC, USA) and R version 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 125 patients (mean age 68.4 ± 9.5 years) were included for analysis in this study. Baseline characteristics, angiographic appearance, IVUS measurements, and iFR values are as described in Table 1. Most patients were male (84.8%), with 56.8% of Asian ethnicity. Two patients had significant left ventricular impairment at presentation (1.6%). Eighty-nine (71.2%) patients were found to have distal left main disease. Ostial left anterior descending artery (LAD) and/or left circumflex artery (LCX) lesions were present in 56 patients (44.8%). Mean QCA-derived % stenosis of all lesions was 46.3 ± 13.4% stenosis.
Table 1:
Clinical characteristics, angiographic, imaging, and physiologic parameters.
| Total (N=125) | |
|---|---|
| Clinical characteristics | |
| Age (years) | 68.4 ± 9.5 |
| Male Sex (%) | 106 (84.8) |
| Asian (%) | 71 (56.8) |
| Hypertension (%) | 93 (74.4) |
| Hyperlipidemia (%) | 100 (80.0) |
| Diabetes mellitus (%) | 42 (33.6) |
| Chronic kidney disease (%) | 38 (30.4) |
| Smoking (%) | 47 (37.6) |
| Family history (%) | 29 (23.2) |
| Previous myocardial infarction (%) | 35 (28.0) |
| Left ventricular ejection fraction <30% (%) | 2 (1.6) |
| Height (cm) | 165.6 ± 10.5 |
| Weight (Kg) | 68 (61 - 87) |
| Body surface area (m2) | 1.75 (1.62 - 2.00) |
| Angiographical parameters | |
| Location of left main disease | |
| ostial | 47 (37.6) |
| mid | 40 (32.0) |
| distal | 89 (71.2) |
| QCA %stenosis (%) | 46.3 ± 13.4 |
| IVUS parameters | |
| Minimum lumen area (mm2) | 4.99 (3.74 - 6.95) |
| Minimum lumen area/BSA (mm2/m2) | 2.77 (2.17 - 3.80) |
| Lumen area stenosis (%) | 48.9 ± 17.2 |
| Atheroma burden (%) | 72.5 (63.3 - 78.2) |
| Physiological parameters | |
| iFR | 0.87 (0.78 - 0.93) |
| Positive iFR (≤0.89) | 75 (60.0) |
Abbreviations: QCA: Quantitative coronary angiography, IVUS: Intravascular Ultrasound, iFR: instantaneous wave-free ratio
Median MLA (IQR), mean lumen area stenosis, and median atheroma burden (IQR) were 4.99 (3.74-6.95) mm2, 48.9 ± 17.2%, and 72.5 (63.3-78.2) %, respectively. Median iFR (IQR) was 0.87 (0.78-0.93) and the iFR was ≤0.89 in 75 patients (60.0%). The receiver operating curve analysis (Figure 1A) showed that an iFR of ≤0.89 identified MLA <6 mm2 with an area under the curve of 0.77 with a sensitivity and specificity of 77% and 66% respectively (P <0.0001). Among the 69 patients without ostial LAD or LCX disease, receiver operating curve analysis showed that an iFR of ≤0.89 identified MLA <6 mm2 with an area under the curve of 0.84, with sensitivity and specificity of 70% and 84% respectively (P <0.0001) (Figure 1B). Positive and negative predictive value of using iFR of ≤0.89 to identify MLA <6 mm2 were 0.77 and 0.66 in all patients, and 0.84 and 0.71 in those without ostial disease (Figure 1C, 1D). Direct correlation of IVUS MLA and iFR revealed a correlation coefficient of 0.42 (P <0.0001) (Figure 1E, Table 2). IVUS MLA correlated with simultaneously measured FFR (N = 75) with a correlation coefficient of 0.32 (P = 0.01) (Supplemental Table I), and there was a moderate positive correlation between iFR and FFR values (correlation coefficient r = 0.67, P <0.0001). However, discordance between iFR and FFR was observed in 16 patients (21%).
Figure 1:

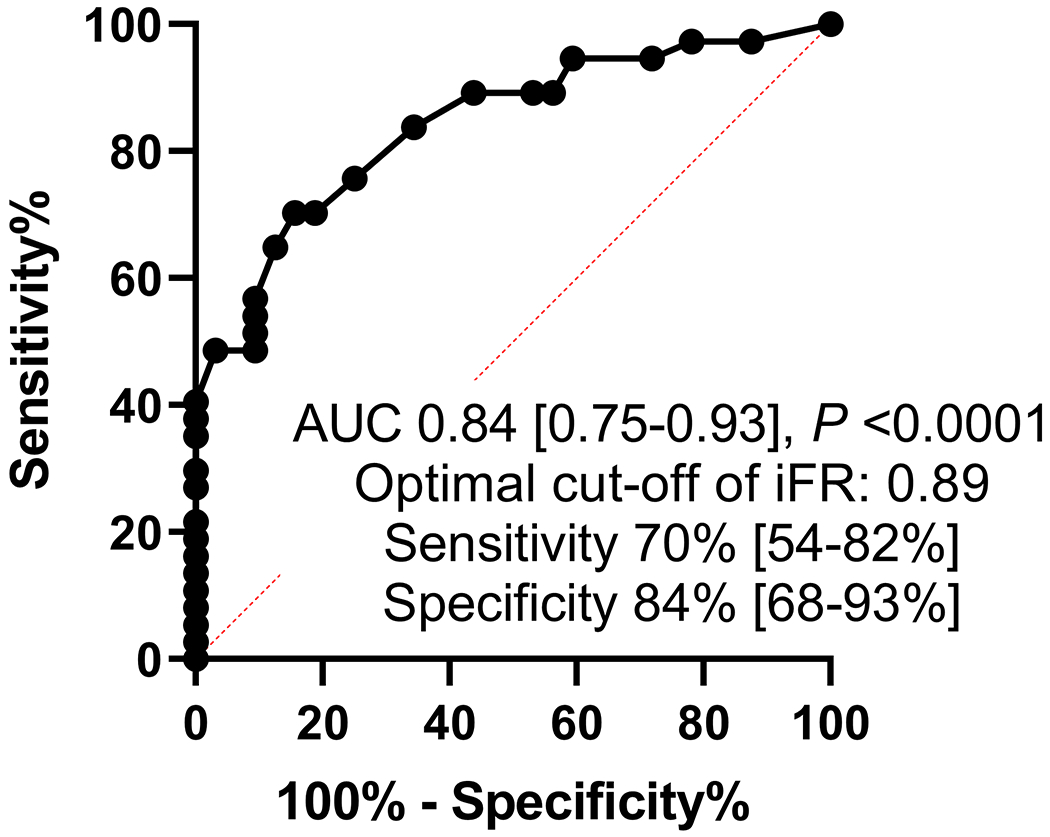
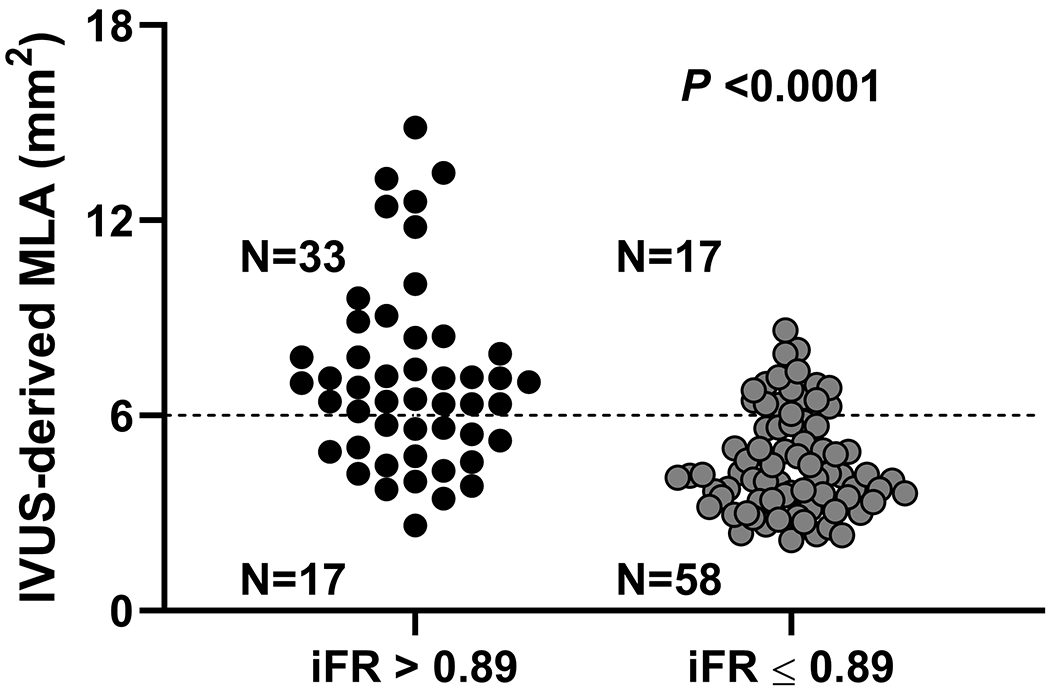
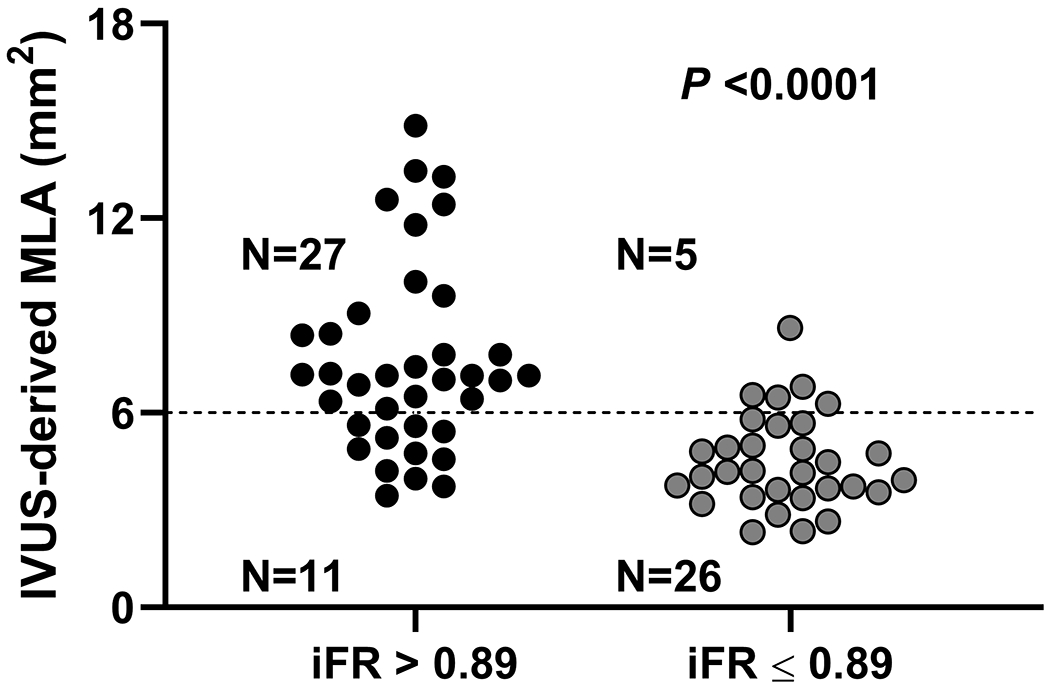
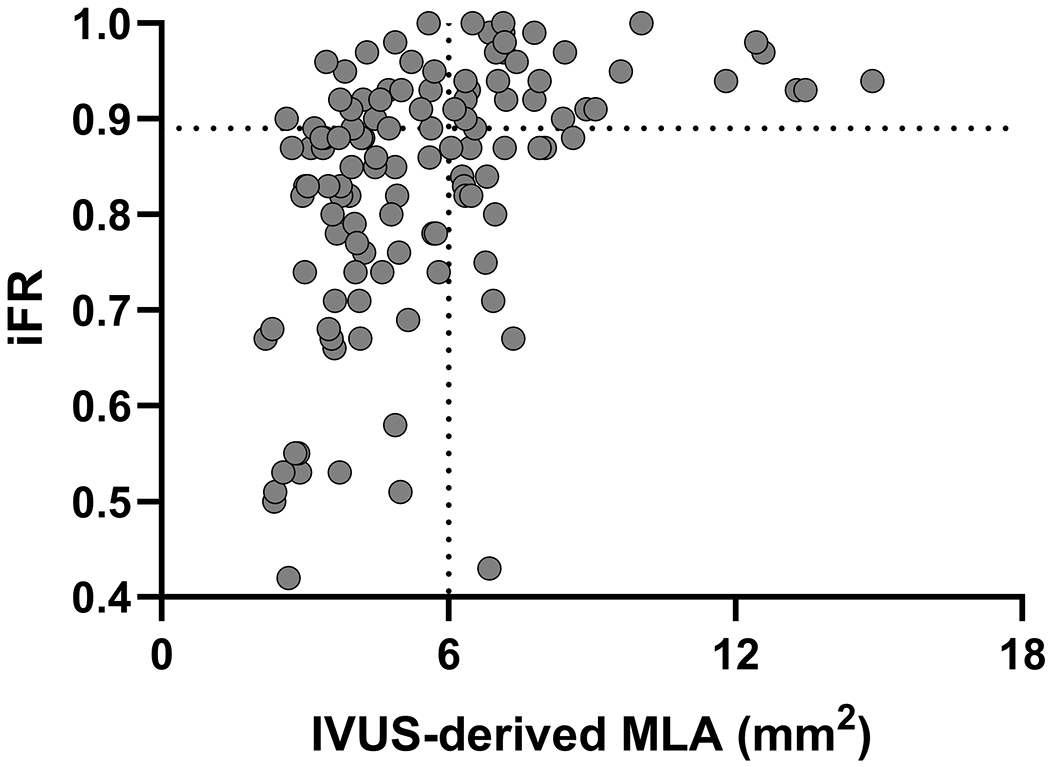
Receiver operating curves for correlation of iFR for prediction of MLA <6 mm2. Legend: A and C: All patients included in the analysis. B and D exclude ostial left anterior descending and left circumflex artery disease from the analysis. E. Scatter plot showing the correlation between iFR and MLA.
Abbreviations: iFR: instantaneous wave-free ratio, IVUS: intravascular ultrasound, MLA: minimum lumen area
Table 2:
Correlation between IVUS-derived parameters and iFR
| IVUS-derived value | Correlation with iFR |
||
|---|---|---|---|
| Correlation coefficient | P value | ||
| MLD (mm) | 2.19 ± 0.68 | 0.41 | <0.0001 |
| MLA (mm2) | 4.99 (3.74 – 6.95) | 0.42 | <0.0001 |
| Area stenosis (%) | 48.9 ± 17.2 | −0.38 | <0.0001 |
| MLA/BSA (mm2/m2) | 2.77 (2.17 – 3.80) | 0.40 | <0.0001 |
Abbreviations: IVUS: Intravascular ultrasound, iFR: instantaneous wave free ratio, MLD: Minimum Lumen Diameter, MLA: Minimum Lumen Area, BSA: Body Surface Area
To evaluate the influence of BSA on our results, we separated our patients by median BSA (1.75 mm2) into 2 groups. 64 patients had a BSA <1.75 mm2 (78% Asian) and 61 patients had a BSA ≥1.75 mm2 (34% Asian). 17 out of 64 patients in the BSA <1.75 mm2 group had a normal iFR while 47 had an abnormal iFR. 33 out of 61 patients in the BSA ≥ 1.75 mm2 group had a normal iFR and 28 had an abnormal iFR. The receiver operating curve analysis showed that in patients with BSA <1.75 mm2, an iFR of ≤0.89 was the optimal cutoff for the identification of an MLA <6 mm2 with an area under the curve of 0.74 (P=0.003), with a sensitivity of 85% and specificity of 59% (Figure 2A). In patients with a BSA ≥1.75 mm2, an iFR of 0.86 was the optimal cutoff for the identification of an MLA <6 mm2 with an area under the curve of 0.72 (P=0.003) with a sensitivity of 57% and specificity of 85%. The sensitivity and specificity were 64% and 70% respectively when a cutoff of ≤0.89 was used (Figure 2B). This shows that, in this study, BSA did not have a major impact in defining the best cutoff for iFR for patients with LM disease.
Figure 2:
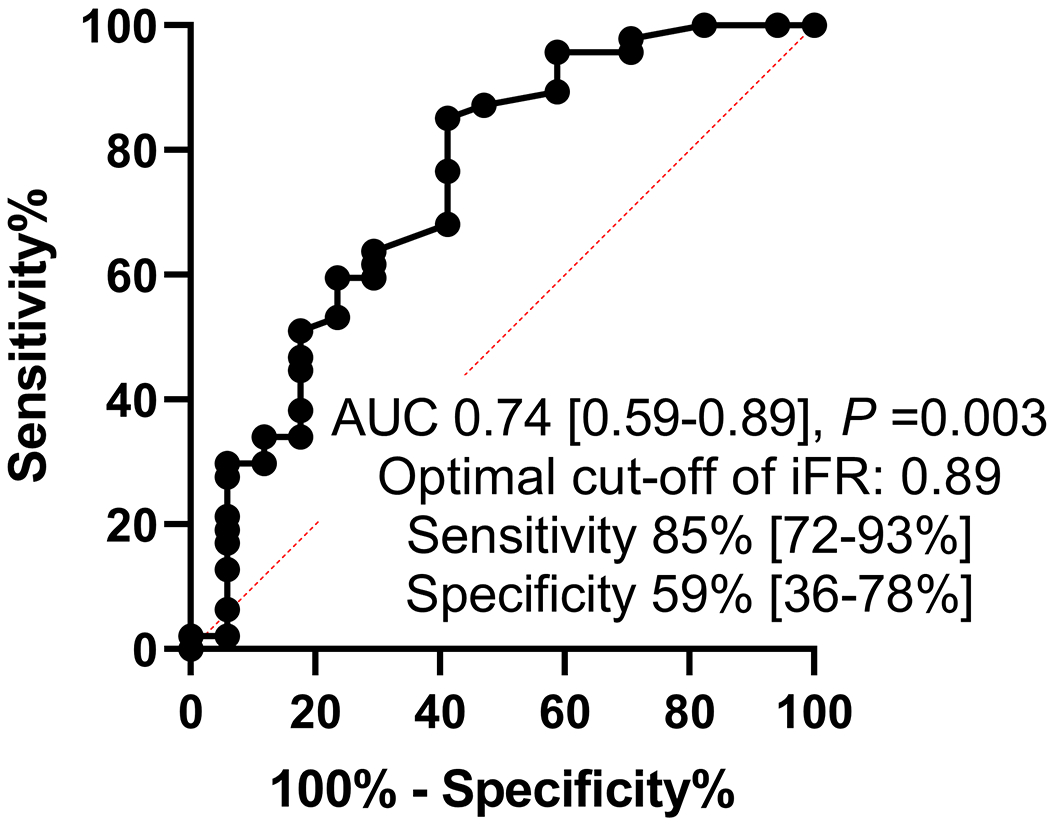

Receiver operating curve analysis for optimal cut-off of iFR for prediction of MLA <6 mm2 divided into 2 body surface groups separated by the median. Legend: A includes patients with BSA <1.75 and B includes patients with BSA ≥1.75.
Abbreviations: BSA: Body surface area, iFR: instantaneous wave-free ratio, MLA: minimum lumen area.
We repeated our analysis to evaluate correlation between indexed MLA/BSA and iFR ≤0.89. Median MLA/BSA (IQR) was 2.77 (2.17-3.80) mm2/m2. The receiver operating curve analysis (Figure 3A) showed than an MLA/BSA of 2.86 mm2/m2 correlated to an iFR ≤0.89 with an area under the curve of 0.75 (P <0.0001) with a sensitivity of 70% and specificity of 68%. When ostial LAD and LCX disease was excluded, the receiver operating curve analysis (Figure 3B) showed than an MLA/BSA of 2.86 mm2/m2 correlated to an iFR ≤0.89 with an area under the curve of 0.82 (P <0.0001) with a sensitivity of 77% and specificity of 76%. Correlation of indexed IVUS MLA/BSA and iFR showed a correlation coefficient of 0.40 (P <0.0001).
Figure 3:

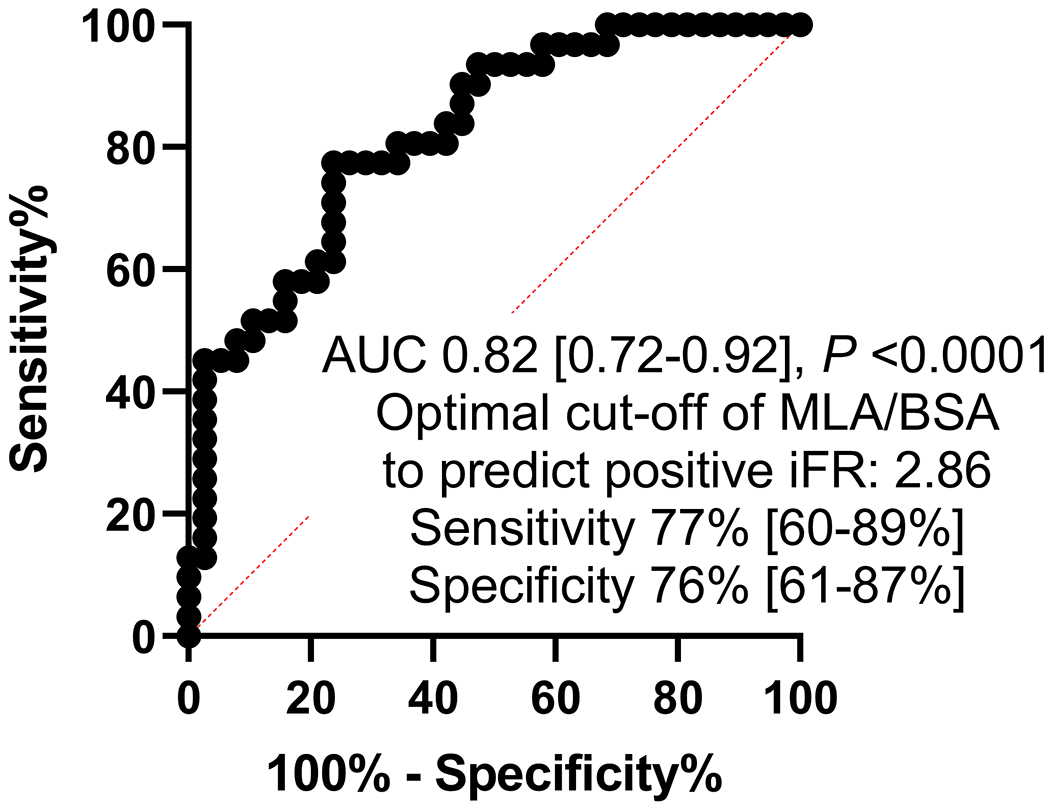
Receiver operating curve analysis for indexed MLA to BSA for prediction of positive iFR (≤0.89). Legend: A includes all patients included in the analysis. B excludes ostial left anterior descending and left circumflex artery disease from the analysis.
Abbreviations: ROC: Receiver operating Curve, MLA: Minimum Lumen area, BSA: Body surface area, iFR: instantaneous wave-free ratio.
Discussion
Our study demonstrates that for LMCD, iFR of ≤0.89 correlates well with IVUS MLA of <6 mm2; this includes patients with ostial LAD or LCX disease. This suggests that the use of iFR can be a viable adjunctive test in the evaluation of intermediate LMCD.
The evaluation of LMCD is a difficult challenge. Traditionally, angiographic stenosis of 50% or greater has been used as the cutoff for significant obstruction. This is unfortunately subject to significant inter-observer variability, with studies showing less than 50% concordance in visual assessment of intermediate LMCD lesions among experienced operators 1. As a result, adjunctive diagnostic evaluation is necessary. Stenosis percentage as determined by QCA did not correlate to iFR values and as previously shown does not predict physiologic significance of LMCD 16.
FFR has been well established in the evaluation of the physiologic significance of intermediate coronary lesions, with multiple randomized control trials shaping its use in coronary interventions today 17–19. However, these trials excluded LMCD, and the application of FFR guided intervention of LMCD are based mostly on a retrospective nonrandomized study of 213 patients 9. Additionally, the use of FFR is limited when faced with the high prevalence of downstream LAD and or LCX lesions, iFR pullback however may allow for improved pre-intervention assessment of tandem lesions 20, 21. The assessment of tandem lesions remains difficult with both modalities in the setting of distal LMCD. FFR limitations also include side effects with administration of adenosine as well as false negative measurements secondary to failure to induce hyperemia; this is avoided with iFR as it is a resting, hyperemia-independent index. Inaccurate measurements secondary to guide dampening in ostial lesions during normalization as well as recording are limitations to both iFR and FFR, and can be avoided with attention to technique. Deferral of intervention based on IVUS MLA is based on an anatomic measure that may be influenced by BSA with various studies concluding a cutoff of MLA ranging from 4.5 mm2 to 7.5 mm2. An MLA of 6 mm2 is the recommended cutoff and was prospectively validated in 354 patients in the LITRO study 8. In the present study, we found that IVUS MLA and iFR demonstrated a significant positive correlation, which was not improved by accounting for BSA.
Two pivotal studies supporting the non-inferiority of iFR to FFR in terms of safety, the DEFINE-FLAIR and iFR SWEDEHEART trials, did not include LMCA stenoses. However, recent studies support the value of iFR as a decision-making tool in this setting. Overall, an 81% concordance in positive iFR and FFR values in LM disease, using an iFR cutoff of 0.89 and FFR cutoff of 0.80, was found by De Rosa et al 22. More importantly, using a study design similar to those supporting the value of FFR in the LM, the DEFINE-LM study found that deferring revascularization of LM lesions with iFR >0.89 was as safe as performing PCI in those with iFR ≤0.89 14. Our study provides in this regard complementary information, suggesting that iFR may also reflect severity of LM stenoses with an IVUS MLA similar to that found previously for FFR (6 mm2) and used in studies supporting IVUS as a guiding tool in this specific coronary location 8.
Study Limitations
This was a retrospective cohort analysis, and the lack of randomization may have allowed potential for selection bias in the patients who underwent evaluation with iFR and IVUS. Additionally, this study was designed with IVUS MLA of 6 mm2 as the standard measurement for significance of obstruction, which may not be an optimal cutoff value for every patient. We attempted to account for this limitation by also correlating iFR to an indexed IVUS MLA/BSA, which showed similar results; however, we could not account for all the possible confounders such as sex, race, and myocardial mass because of a small sample size and/or lack of data 23. Also, IVUS pullback was manually performed in 22 patients, leaving a potential risk of missing true MLA. Finally, this study was not designed to evaluate major adverse cardiovascular events, but rather correlation of diagnostic modalities used in a real world setting to help guide the clinician. The presence of severe downstream stenoses in LAD/LCX (>85-90% diameter stenosis) may potentially predispose to underestimating the pressure gradient and overestimating the iFR value by decreasing resting coronary flow 24, 25; nevertheless, we demonstrated that iFR of ≤0.89 correlates well with IVUS MLA of 6 mm2, reflecting the real-world use of iFR in clinical practice to guide LMCD management.
Conclusion
In patients with angiographically intermediate left main coronary artery disease, an iFR cutoff of 0.89 positively correlates with an IVUS cutoff of 6 mm2. This may further support the use of iFR with a cutoff point of 0.89 to guide management of patients with intermediate left main disease, requiring further validation. Also, future studies are necessary to investigate how we can integrate iFR with IVUS findings to make a critical decision whether to revascularize intermediate left main disease or not.
Supplementary Material
What is known.
The evaluation of left main coronary artery lesions is a difficult challenge.
iFR use is growing, however iFR of the left main has not been well studied.
What the Study Adds.
This study finds that iFR ≤0.89 can be used in the evaluation of angiographically intermediate left main coronary disease, and correlates well with accepted IVUS MLA cutoffs.
The correlation is not significantly different when BSA was taken into account.
iFR can add information in the challenging evaluation of left main disease.
Acknowledgments:
Sources of Funding:
This study was partly supported by the National Institute of Health [grant numbers DK120292, DK 122734] and the Mayo Foundation.
Disclosures:
TW has received consulting fees from Abbott Vascular and Philips. CMC has received speaker’s honoraria from Philips Volcano. HS has received a research grant from Amgen. JPH is supported by the Wellcome Trust (212183/Z/18/Z). YK reports speaker fees from Abbott Vascular and Philips. YA and SS are supported by the Academy of Medical Sciences and Imperial Biomedical Research Centre. SS (G1000357) is supported by the Medical Research Council; and has served on the Speakers Bureau and participated in educational events for Pfizer, Phillips, Daichi-Sankyo, and AstraZeneca; and has received speaker fees from Volcano, Pfizer, and Medtronic. RAL has received a speaker honorarium from Philips Volcano. JE has served as consultant and speaker for Philips Volcano. RP has served as a consultant for and received speaker fees from Philips Volcano; and is supported by the British Heart Foundation (FS/11/46/28861). SNijjer has received travel support and speaker fees from Philips Volcano. JED holds patents pertaining to the iFR technology. JED is a consultant for Philips Volcano and has received research grants from Philips Volcano. All other authors declare no conflicts of interest
Abbreviations List
- BSA
body surface area
- FFR
fractional flow reserve
- iFR
instantaneous wave-free ratio
- IVUS
intravascular ultrasound
- IQR
interquartile range
- LAD
left anterior descending
- LCX
left circumflex
- LMCD
left main coronary disease
- MLA
minimum lumen area
- QCA
quantitative coronary analysis
References
- 1.Lindstaedt M, Spiecker M, Perings C, Lawo T, Yazar A, Holland-Letz T, Muegge A, Bojara W and Germing A. How good are experienced interventional cardiologists at predicting the functional significance of intermediate or equivocal left main coronary artery stenoses? Int J Cardiol. 2007;120:254–261. [DOI] [PubMed] [Google Scholar]
- 2.Puri R, Kapadia SR, Nicholls SJ, Harvey JE, Kataoka Y and Tuzcu EM. Optimizing outcomes during left main percutaneous coronary intervention with intravascular ultrasound and fractional flow reserve: the current state of evidence. JACC Cardiovasc Interv. 2012;5:697–707. [DOI] [PubMed] [Google Scholar]
- 3.Bech GJ, Droste H, Pijls NH, De Bruyne B, Bonnier JJ, Michels HR, Peels KH and Koolen JJ. Value of fractional flow reserve in making decisions about bypass surgery for equivocal left main coronary artery disease. Heart (British Cardiac Society). 2001;86:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Courtis J, Rodes-Cabau J, Larose E, Potvin JM, Dery JP, Larochelliere RD, Cote M, Cousterousse O, Nguyen CM, Proulx G, et al. Usefulness of coronary fractional flow reserve measurements in guiding clinical decisions in intermediate or equivocal left main coronary stenoses. The American journal of cardiology. 2009;103:943–949. [DOI] [PubMed] [Google Scholar]
- 5.Fassa AA, Wagatsuma K, Higano ST, Mathew V, Barsness GW, Lennon RJ, Holmes DR Jr., Lerman A Intravascular ultrasound-guided treatment for angiographically indeterminate left main coronary artery disease: a long-term follow-up study. J Am Coll Cardiol. 2005;45:204–211. [DOI] [PubMed] [Google Scholar]
- 6.Lee CH, Tai BC, Soon CY, Low AF, Poh KK, Yeo TC, Lim GH, Yip J, Omar AR, Teo SG, et al. New set of intravascular ultrasound-derived anatomic criteria for defining functionally significant stenoses in small coronary arteries (results from Intravascular Ultrasound Diagnostic Evaluation of Atherosclerosis in Singapore [IDEAS] study). The American journal of cardiology. 2010;105:1378–1384. [DOI] [PubMed] [Google Scholar]
- 7.Kang SJ, Lee JY, Ahn JM, Song HG, Kim WJ, Park DW, Yun SC, Lee SW, Kim YH, Mintz GS, et al. Intravascular ultrasound-derived predictors for fractional flow reserve in intermediate left main disease. JACC Cardiovasc Interv. 2011;4:1168–1174. [DOI] [PubMed] [Google Scholar]
- 8.de la Torre Hernandez JM, Hernandez Hernandez F, Alfonso F, Rumoroso JR, Lopez-Palop R, Sadaba M, Carrillo P, Rondan J, Lozano I, Ruiz Nodar JM, et al. Prospective application of pre-defined intravascular ultrasound criteria for assessment of intermediate left main coronary artery lesions results from the multicenter LITRO study. J Am Coll Cardiol. 2011;58:351–358. [DOI] [PubMed] [Google Scholar]
- 9.Hamilos M, Muller O, Cuisset T, Ntalianis A, Chlouverakis G, Sarno G, Nelis O, Bartunek J, Vanderheyden M, Wyffels E, et al. Long-term clinical outcome after fractional flow reserve-guided treatment in patients with angiographically equivocal left main coronary artery stenosis. Circulation. 2009;120:1505–1512. [DOI] [PubMed] [Google Scholar]
- 10.Stone GW, Sabik JF, Serruys PW, Simonton CA, Généreux P, Puskas J, Kandzari DE, Morice MC, Lembo N, Brown WM 3rd, et al. Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. The New England journal of medicine. 2016;375:2223–2235. [DOI] [PubMed] [Google Scholar]
- 11.Park SJ, Ahn JM, Kang SJ, Yoon SH, Koo BK, Lee JY, Kim WJ, Park DW, Lee SW, Kim YH, et al. Intravascular ultrasound-derived minimal lumen area criteria for functionally significant left main coronary artery stenosis. JACC Cardiovasc Interv. 2014;7:868–874. [DOI] [PubMed] [Google Scholar]
- 12.Davies JE, Sen S, Dehbi HM, Al-Lamee R, Petraco R, Nijjer SS, Bhindi R, Lehman SJ, Walters D, Sapontis J, et al. Use of the Instantaneous Wave-free Ratio or Fractional Flow Reserve in PCI. The New England journal of medicine. 2017;376:1824–1834. [DOI] [PubMed] [Google Scholar]
- 13.Gotberg M, Christiansen EH, Gudmundsdottir IJ, Sandhall L, Danielewicz M, Jakobsen L, Olsson SE, Ohagen P, Olsson H, Omerovic E, et al. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. The New England journal of medicine. 2017;376:1813–1823. [DOI] [PubMed] [Google Scholar]
- 14.Warisawa T, Cook CM, Rajkumar C, Howard JP, Seligman H, Ahmad Y, El Hajj S, Doi S, Nakajima A, Nakayama M, et al. Safety of Revascularization Deferral of Left Main Stenosis Based on Instantaneous Wave-Free Ratio Evaluation. JACC Cardiovasc Interv. 2020;13:1655–1664. [DOI] [PubMed] [Google Scholar]
- 15.Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, Pinto FJ, Rosenfield K, Siegel RJ, Tuzcu EM, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:1478–1492. [DOI] [PubMed] [Google Scholar]
- 16.Jasti V, Ivan E, Yalamanchili V, Wongpraparut N and Leesar MA. Correlations between fractional flow reserve and intravascular ultrasound in patients with an ambiguous left main coronary artery stenosis. Circulation. 2004;110:2831–2836. [DOI] [PubMed] [Google Scholar]
- 17.Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van’t Veer M, Bar F, Hoorntje J, Koolen J, Wijns W, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105–2111. [DOI] [PubMed] [Google Scholar]
- 18.van Nunen LX, Zimmermann FM, Tonino PA, Barbato E, Baumbach A, Engstrom T, Klauss V, MacCarthy PA, Manoharan G, Oldroyd KG, et al. Fractional flow reserve versus angiography for guidance of PCI in patients with multivessel coronary artery disease (FAME): 5-year follow-up of a randomised controlled trial. Lancet (London, England). 2015;386:1853–60. [DOI] [PubMed] [Google Scholar]
- 19.Fearon WF, Nishi T, De Bruyne B, Boothroyd DB, Barbato E, Tonino P, Juni P, Pijls NHJ and Hlatky MA. Clinical Outcomes and Cost-Effectiveness of Fractional Flow Reserve-Guided Percutaneous Coronary Intervention in Patients With Stable Coronary Artery Disease: Three-Year Follow-Up of the FAME 2 Trial (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation). Circulation. 2018;137:480–487. [DOI] [PubMed] [Google Scholar]
- 20.Nijjer SS, Sen S, Petraco R, Mayet J, Francis DP and Davies JE. The Instantaneous wave-Free Ratio (iFR) pullback: a novel innovation using baseline physiology to optimise coronary angioplasty in tandem lesions. Cardiovascular revascularization medicine : including molecular interventions. 2015;16:167–171. [DOI] [PubMed] [Google Scholar]
- 21.Kikuta Y, Cook CM, Sharp ASP, Salinas P, Kawase Y, Shiono Y, Giavarini A, Nakayama M, De Rosa S, Sen S, et al. Pre-Angioplasty Instantaneous Wave-Free Ratio Pullback Predicts Hemodynamic Outcome In Humans With Coronary Artery Disease: Primary Results of the International Multicenter iFR GRADIENT Registry. JACC Cardiovasc Interv. 2018;11:757–767. [DOI] [PubMed] [Google Scholar]
- 22.De Rosa S, Polimeni A, De Velli G, Conte M, Sorrentino S, Spaccarotella C, Mongiardo A, Sabatino J, Contarini M and Indolfi C. Reliability of Instantaneous Wave-Free Ratio (iFR) for the Evaluation of Left Main Coronary Artery Lesions. J Clin Med. 2019;8:1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson TW, Räber L, di Mario C, Bourantas C, Jia H, Mattesini A, Gonzalo N, de la Torre Hernandez JM, Prati F, Koskinas K, et al. Clinical use of intracoronary imaging. Part 2: acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: an expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. European heart journal. 2019;40:2566–2584. [DOI] [PubMed] [Google Scholar]
- 24.Gould KL, Lipscomb K and Calvert C. Compensatory changes of the distal coronary vascular bed during progressive coronary constriction. Circulation. 1975;51:1085–94. [DOI] [PubMed] [Google Scholar]
- 25.Nijjer SS, de Waard GA, Sen S, van de Hoef TP, Petraco R, Echavarría-Pinto M, van Lavieren MA, Meuwissen M, Danad I, Knaapen P, et al. Coronary pressure and flow relationships in humans: phasic analysis of normal and pathological vessels and the implications for stenosis assessment: a report from the Iberian-Dutch-English (IDEAL) collaborators. European heart journal. 2016;37:2069–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


