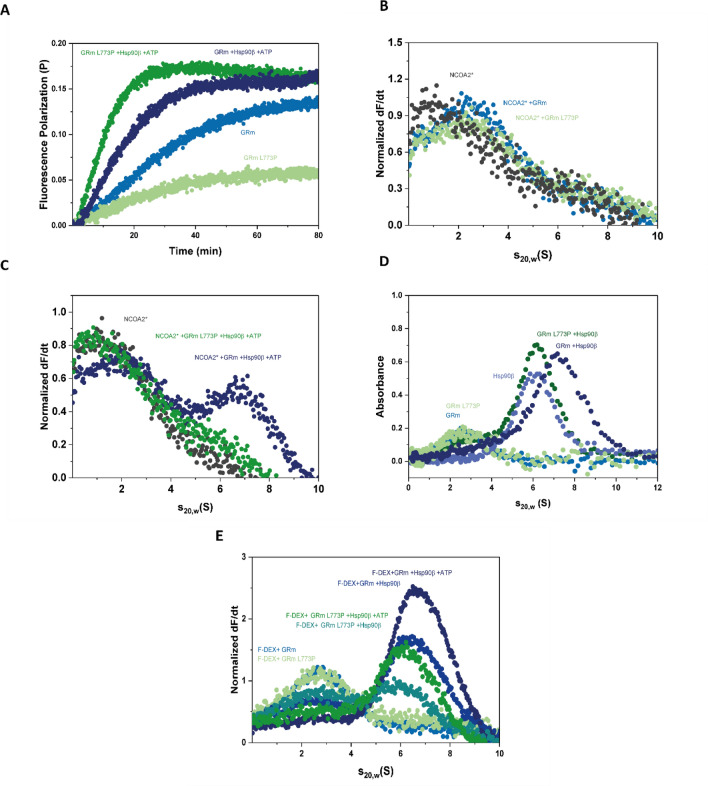
Figure 2.
GRm L773P exhibits reduced hormone binding that is restored by Hsp90β and altered binding to a coactivator-derived peptide. (A) Fluorescence polarization kinetics show that Hsp90β is able to restore hormone binding to GRm L773P (olive). (B,C) Sedimentation velocity AUC coupled to fluorescence optics shows the binding of GRm and GRm L773P to a NCOA2-derived peptide in the presence and absence of Hsp90β. Only GRm is able to form a ternary complex with Hsp90β and the NCOA-2 peptide. (D) Sedimentation velocity AUC coupled to absorbance optics shows GRm and GRm L773P binding to Hsp90β in 1:1 stoichiometry in the absence of ATP. (E) Sedimentation velocity AUC following F-DEX shows that GRmL773P∙Hsp90β binds hormone to a lower extent than GRm, both in the absence and presence of ATP, but both GR·Hsp90β assemblies are competent in hormone-binding.