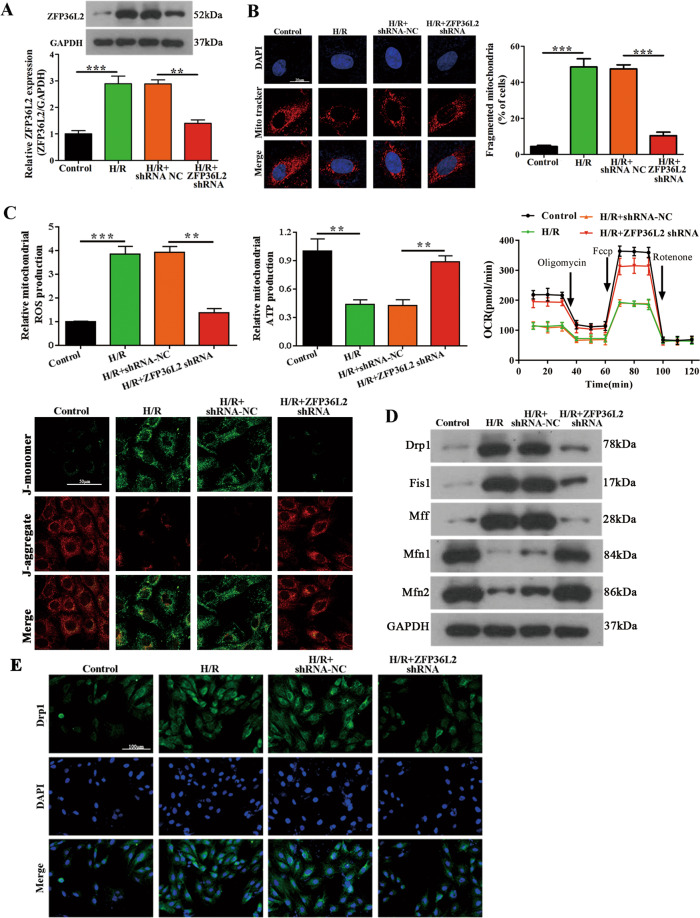
Fig. 2. ZFP36L2 knockdown suppresses hypoxia/reoxygenation (H/R) injury and alleviates mitochondrial fusion and fission in vitro.
A A H/R injury model was established by inducing hypoxia for 4 h followed by reoxygenation for 1 h. The mRNA and protein expression of ZFP36L2 in cardiomyocytes were determined by qRT-PCR and western blot, respectively (n = 3). B Representative micrographs of cardiomyocytes after H/R injury expressing normal control shRNA or shRNA against ZFP36L2 stained with MitoTracker Red (mitochondria) and 4′, 6-diamidino-2-phenylindole (DAPI) (nucleus). Quantification of cells with fragmented mitochondria was detected under different conditions. At least 100 cells were counted per condition (Scale bar = 20 μm) (n = 3). C The levels of ROS, ATP and OCR were measured to evaluate mitochondrial function in H/R-treated cardiomyocytes, which was transfected with ZFP36L2 shRNA or shRNA NC. JC-1staining (2 µg/ml) was used to analyze mitochondrial membrane potential (△Ψm) (Scale bar = 50 μm) (n = 3). D Expression of mitochondrial fusion-related genes Drp1, Fis1, Mff, Mfn1, and Mfn2 after cardiomyocytes H/R treatment was evaluated by western blot (n = 3). E Representative micrographs from immunofluorescence assays of cardiomyocytes stained for Drp1 (green) and the nucleus (DAPI) (Scale bar = 100 μm) (n = 3). **P < 0.01; ***P < 0.001.