Fig. 2.
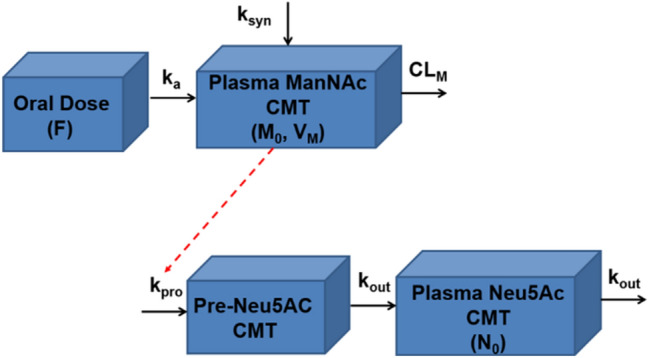
Diagram of final structural pharmacokinetic model for ManNAc and Neu5Ac. F oral bioavailability, ka first-order oral absorption rate-constant for exogenously administered ManNAc (h−1), ksyn zero-order endogenous production rate constant for ManNAc (μg/h), CMT compartment, CLM apparent clearance for ManNAc (L/h), M0 initial endogenous plasma ManNAc concentration (ng/mL), VM apparent volume of distribution for ManNAc (L), kpro zero-order production rate constant for Neu5Ac in precursor compartment (ng/mL·h), Pre-Neu5Ac Neu5Ac precursor, kout first-order elimination rate constant for Neu5Ac (h−1), N0 initial endogenous plasma Neu5Ac concentration (ng/mL)
