Figure 7. Implanted Human α Cells Proliferate in Mice Dosed with GCGR Antibody.
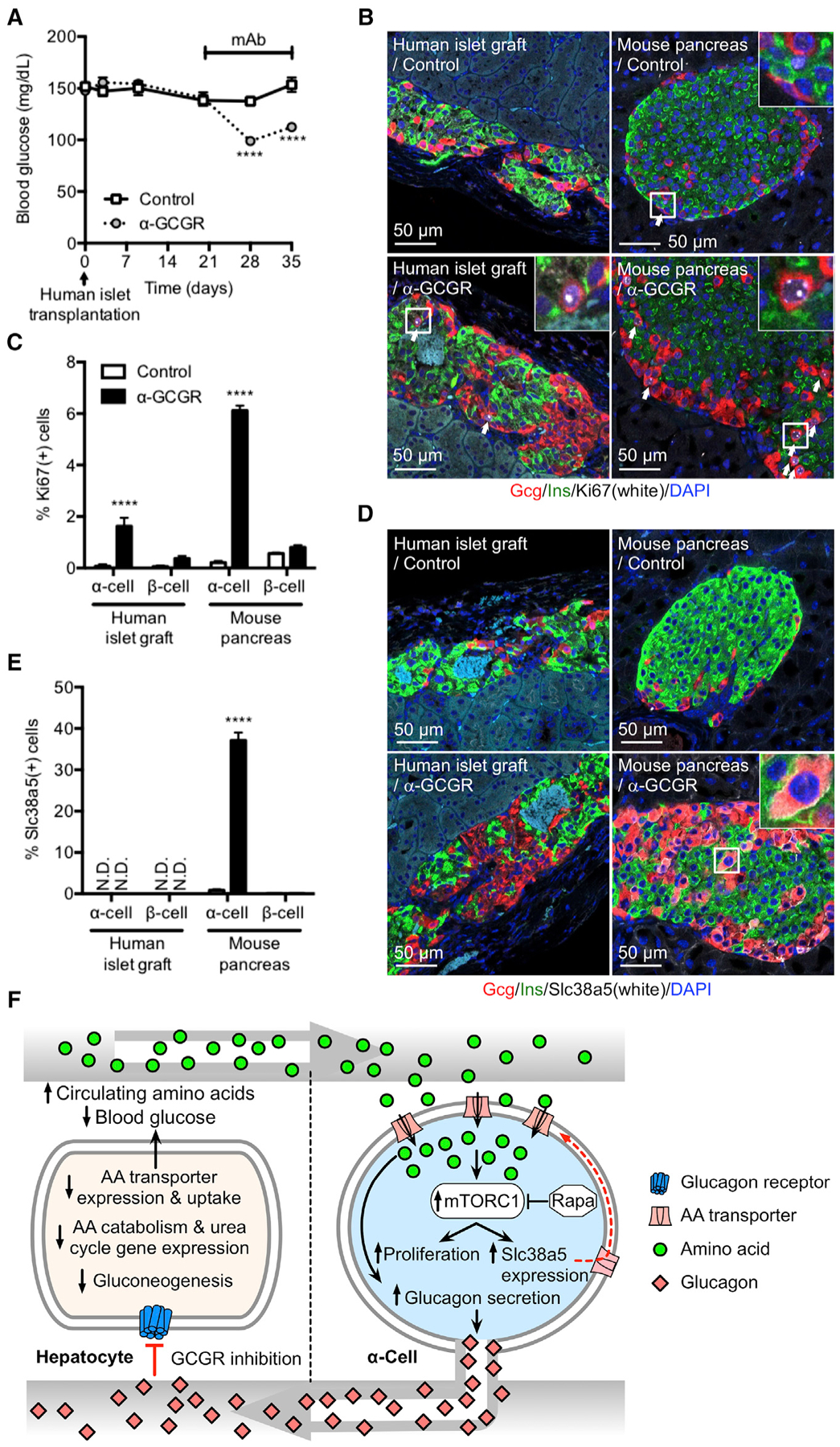
(A) Blood glucose in immune-deficient SCID mice implanted with 1,000 IEQ human islets under the kidney capsule for 21 days before dosed weekly with either control or GCGR-blocking antibody. Data are mean ± SEM, n = 8–9. ****p < 0.0001.
(B) Glucagon (red), insulin (green), Ki67 (white), and DAPI nuclei (blue) immunofluorescence staining of representative sections of human islet graft and pancreas from mice implanted and antibody treated as described in (A).
(C) KI67-positive α and β cells in human islet grafts and pancreas from mice implanted and antibody treated as described in (A). Data are mean ± SEM, n = 8–9. ****p < 0.0001.
(D) Glucagon (red), insulin (green), Slc38a5 (white), and DAPI nuclei (blue) immunofluorescence staining of representative sections of human islet graft and pancreas from mice implanted and antibody treated as described in (A).
(E) SLC38A5-positive α and β cells in human islet grafts and pancreas from mice implanted and antibody treated as described in (A). Data are mean ± SEM, n = 8–9. ****p < 0.0001. N.D., not detected.
(F) Model for liver-pancreatic α cell axis. Glucagon is released from α cells and acts on glucagon receptors in the liver to stimulate amino acid uptake, metabolism, and gluconeogenesis to promote hepatic glucose output raising blood glucose. When glucagon action in the liver is inhibited or disrupted, it leads to decreased amino acid uptake and metabolism resulting in elevated circulating amino acid levels. Gluconeogenesis is inhibited leading to reduction in blood glucose. The α cell senses increased plasma amino acids to stimulate glucagon secretion and mTOR-dependent proliferation. mTOR also stimulates expression of Slc38a5 to promote further amino acid uptake.
