Abstract
Background & Aims
Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) is responsible for coronavirus disease 2019 (COVID‐19), which in males, especially in advanced age, can sometimes evolve into acute respiratory distress syndrome. In addition, mild to moderate alterations in liver function tests (LFTs) have been reported in the worst affected patients. Our review aims to analyse data on the incidence and prognostic value of LFT alterations, the underlying mechanisms and the management of pre‐existing liver disease in COVID‐19 affected patients.
Methods
We searched available literature through online PubMed database using terms as “SARS‐CoV‐2,” “Liver damage,” “Liver Function tests,” “COVID‐19,” “pre‐existing liver disease,” “drug‐induced liver injury.”
Results
Available evidence suggest that there could be a relationship between SARS‐CoV‐2 infection and liver damage, although the underlying involved mechanism remains unclear. Cohort studies have shown that high ALT levels, low platelet counts and low albumin levels at admission and during hospitalisation are associated with a high mortality rate. Unfortunately, little is known about the impact of COVID‐19 on pre‐existing liver damage. While chronic viral infections or NAFLD are associated with an increased risk of COVID‐19 progression, patients with cirrhosis may have increased susceptibility to SARS‐CoV‐2 infection due to their systemic immunocompromised status. DILI seems common among hospitalised patient with severe pneumonia.
Conclusion
Mild to moderate liver impairment during Covid‐19 is common, especially in patients with pre‐existing liver disease. Further studies should be performed in order to understand how pre‐existing liver conditions may influence and worsen progression of liver disease in COVID‐19 patients.
Keywords: COVID‐19, Liver Function tests (LTFs), liver injury, SARS‐CoV‐2
1. INTRODUCTION
The world will remember 2020 as the year of the coronavirus disease 2019 (COVID‐19) pandemic. In fact, in the winter of 2019, a new virus reportedly originating from the Hubei province in China, spread rapidly first to Italy, then France and Spain, causing millions of infections and tens of thousands of deaths. This virus, severe acute respiratory syndrome coronavirus‐2(SARS‐CoV‐2), belonging to the Coronaviridae family, is responsible for an acute respiratory distress syndrome. In addition to the typical respiratory signs and symptoms, mild to moderate alterations in liver function tests (LFTs) have been reported in the worst affected patients. LFT alterations, and in particular aminotransferase (AST and ALT) values, are a frequent manifestation of SARS‐CoV‐2 infection and are associated with an increase in lactic dehydrogenase (LDH) and inflammation markers such as C‐reactive protein and ferritin. Many reports, all from studies conducted in China, show that abnormal liver tests and liver injury, mostly hepatocellular rather than cholestatic, are frequent in patients with COVID‐19, 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 as reported in Table 1. Data from Chinese studies have shown that about 2%‐11% of patients hospitalised for COVID‐19 have one or more liver comorbidities and 14%‐53% of patients show altered LFT values. 2 Cai et al defined liver injury as an alteration in transaminases (AST or ALT) up to 3 X ULN and/or alkaline phosphatase (ALP), gamma‐glutamyl transferase (GGT), total bilirubin up to 2 X ULN, and reported this condition in 5% of patients at admission and in 21.3% during hospitalisation, whereas 76% did not show any dysfunction. 3 These figures emphasise the differences between liver injury and the slight alterations in LFTs, which usually occur in systemic viral infections and may reflect systemic immune activation or inflammation induced by circulating cytokines, without an impairment of liver function.
TABLE 1.
Description of studies assessing liver damage in COVID‐19
| Author | Country | Date of study publication, | No of patients | AST/ALT alteration | Overall mortality | Pre‐existing liver disease | |
|---|---|---|---|---|---|---|---|
| 1 | Fan et al 7 | China |
February 2020 retrospective, single‐center study |
148 |
37.2% had abnormal liver function at hospital admission A significantly higher proportion of patients with abnormal liver function (57.8%) had received lopinavir/ritonavir after admission compared to patients with normal liver function (31.3%) |
N/A | N/A |
| 2 | Liu et al 6 | China |
February 2020 Multicenter study |
32 |
6.2% had abnormal AST 28% had abnormal ALT |
N/A | N/A |
| 3 | Chen et al 45 | China |
February 2020 Retrospective, single‐center study |
99 |
35% had increased AST 28% had increased ALT |
11% |
No liver comorbidities described Digestive system disease 11% |
| 4 | Cai et al 3 | China |
February 2020 Cross‐sectional study |
417 |
76.3% had abnormal liver tests 21.5% had liver injury during hospitalization |
N/A | 1%‐5% NAFLD, alcoholic liver disease, and chronic hepatitis B |
| 5 | Xu et al 9 | China |
February 2020 Retrospective case series |
62 | 16% had increased AST (>40 U/I) | 0% | 11% of liver disease |
| 6 | Huang et al 2 | China |
February 2020 Prospective study |
41 | 37% had increased AST (>40 U/I) | 15% | 2% of liver disease |
| 7 | Zhang et al 4 | China |
March 2020 Group‐control study |
115 |
On admission ‐ AST elevated in 14.7% (mean value 32.14 ± 15.74) ‐ ALT elevated un 9,5% (mean values 41 ± 24.9) Mild vs severe COVID‐19 ‐ ALT (21.22 ± 12.7 vs 37.8 ± 32.17 P < 0.001) ‐ AST (24.39 ± 9.79vs 38.87 ± 22.55 P < 0.001) |
0.87% | 2 patients with HBV were excluded |
| 8 | Cao et al 27 | China |
March 2020 Randomized, controlled, open‐label trial |
199 |
‐ AST elevation in patients overall (20.5%), treated with lopinavir/ritonavir (18.8%), treated with standard care (22%) ‐ ALT elevation in patients overall (41%), treated with lopinavir/ritonavir (36.5%), treated with standard care (45.5%), |
Day 28 mortality 22% |
No liver comorbidities described Diabetes (11%) |
| 9 | Guan et al 1 | China |
April 2020 Retrospective study |
1099 |
AST – ALT increased (> 40 U/L): 18%‐19% if mild COVID disease 39%‐28% if severe disease |
1.4% | 2.1% Hepatitis B infection |
| 10 | Shi et al 8 | China |
April 2020 Descriptive study |
81 |
53% had increased AST (> 40 U/I) |
5% | 9% of liver disease, hepatitis, or cirrhosis |
| 11 | Yang et al 10 | China |
May 2020 Single‐center, retrospective, observational study |
52 |
29% had increased transaminases, with no difference between survivors and non‐survivors |
61% |
No liver comorbidities described |
| 12 | Cavalcanti et al 25 | Brazil |
July 2020 Multicenter, randomized, open‐label, controlled trial |
665 |
Transaminase elevation In patients overall (7.7%) and pts. treated with ‐ Hydroxychloroquine +azithromycin (10.9%), ‐ Hydroxychloroquine (8.5%), ‐ Azithromycin (4%), ‐ Neither Hydroxychloroquine nor azithromycin (3.4%), |
0.8% |
No liver comorbidities described Diabetes (19%), Obesity (15%) |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Severe COVID‐19 disease, however, appears to be more frequently associated with liver injury. Huang observed an elevation in AST of up to 5 X UNL in 8 of the 13 patients admitted to the ICU (62%) and in 7 of the 28 outpatient patients (25%). 2 In a large cohort of 1099 patients, abnormal aminotransferase levels were more frequently found in severe COVID‐19 patients than in non‐severe patients (18% in mild COVID‐19 disease vs 28% in severe disease). 1 Rarely, SARS‐CoV‐2 infection can present as acute hepatitis, with transaminases 10‐20 X ULN at admission, with all other causes of acute hepatitis having been ruled out. 11
Some studies have reported LFT values obtained at admission, during hospitalisation and at discharge to evaluate the detrimental effect on the liver of certain drugs used during hospitalisation. Fan et al showed that 37.2% of patients had abnormal liver function at admission, whereas a significantly higher proportion of patients showed abnormal liver function (57.8%) after receiving lopinavir/ritonavir than other patients not receiving these drugs (31.3%). 7
However, although a number of studies have observed a slight increase in aminotransferases in hospitalised patients, and others have reported the worsening of a pre‐existing liver condition due to the superimposed viral infection and to the hepatotoxicity of the treatment drugs, the relationship between pre‐existing liver disease and COVID‐19 disease has not yet been carefully studied. A percentage of increased risk of SARS‐CoV‐2 infection ranging from 2% to 11%, has been reported in NAFLD, alcohol liver disease or HBV hepatitis, 1 , 2 , 3 , 5 , 11 but it remains to be determined whether patients with cirrhosis and COVID‐19 have an increased risk of mortality due to decompensation, or of developing acute‐on‐chronic liver failure (ACLF) (Table 1).
2. PATHOPHYSIOLOGY OF VIRUS INFECTION
It has been clearly shown that the SARS‐CoV‐2 virus enters cells by binding the angiotensin conversion enzyme‐2 (ACE2) receptor similarly to SARS‐CoV. 12
Although many studies show that COVID‐19 is associated with liver injury, the underlying mechanism is not yet known. However, several hypotheses have been formulated: direct cytopathic effects, immune imbalance and cytokine storm with secondary multi‐organ dysfunction, ischaemia and/or hypoxia‐reperfusion injury and, finally, drug‐induced liver injury (Figure 1).
FIGURE 1.
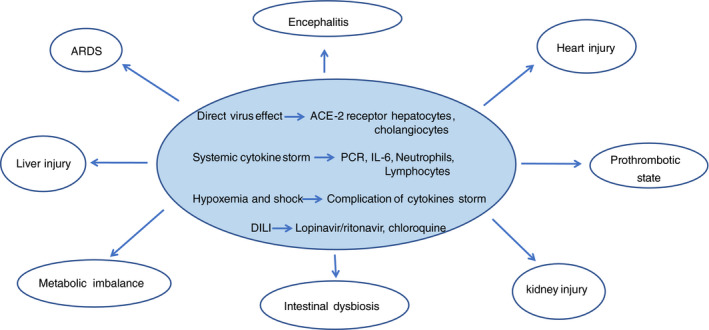
Pathogenetic mechanism of SARS‐CoV 2 infection and multi‐organ involvement cytokine storm
2.1. Direct cytopathic effect
Liver injury in COVID‐19 patients might be directly caused by the viral infection of liver cells. A recent study sequencing RNA on liver tissue samples from four donors who had died from cardio‐circulatory arrest revealed that both hepatocytes and bile duct cells express ACE2 receptors with a high affinity for the spike protein of SARS‐CoV‐2. However, the expression levels of these receptors in cholangiocytes are about 20 times higher than in hepatocytes and comparable to expression in alveolar type II cells. 13 Furthermore, the epithelium of bile ducts plays an important role in liver regeneration and in the regulation of immune response. 14 In cholangiocytes the virus replicates actively and decreases claudin‐1 expression, determining tight junction disruption between cholangiocytes and in turn the impairment of bile acid transport and function. Some studies have seen that ductal cells are usually susceptible to infections, with a consequent impairment of barrier function and the transport of bile acids. 15 Hepatocytes, on the other hand, are not particularly affected by SARS CoV‐2, as there is a reduction in or lack of ACE2 receptors. From this, it can be deduced that liver damage is perhaps not directly linked to the activity of the virus, but there is probably another etiological trigger.
Among the aetiological hypotheses, many authors have argued that liver damage is likely due to the action of immune cells activated in response to the virus and the resulting cytokine activity. 13 In a respiratory viral infection, collateral liver injury has been related to virus‐specific effector cells generated in response to pulmonary infection. Analysis of post‐mortem liver biopsies of patients infected with SARS‐CoV‐2 showed hepatocyte degeneration, focal necrosis, inflammation of bile ducts and portal and peri‐portal inflammation, as well as fat degeneration, 4 but no viral particles were found. The absence of viral particles in the liver, therefore, indicates that liver damage is probably primarily related to the action of activated T cells, in particular Th‐17 and CD8 11 ; a case of microvesicular steatosis was observed with hyperactivation of T cells, reinforcing the hypothesis of immune‐mediated damage rather than a cytopathic effect, as has recently been argued. 5
2.2. Immune imbalance and cytokine storm
Immune dysfunction (including lympho‐penia, a decrease in CD4+ T‐cell levels, and abnormal cytokine levels with cytokine storm) in COVID‐19 patients is associated with disease severity and mortality. 6 High levels of pro‐inflammatory cytokines (TNF‐a, IL‐6, IL‐2, IL‐7, IL‐11, interferon γ, monocyte chemoattractant protein‐1/MCP‐1, and macrophage inflammatory protein 1‐α) are responsible for the cytokine storm, which correlates to increased vascular permeability, multi‐organ failure (MOF), the activation of coagulation pathways and in severe cases, even death. 16 The state of systemic inflammation also causes an imbalance in coagulative homeostasis toward a pro‐coagulant state, which predisposes to the onset of micro‐thrombosis, disseminated intravascular coagulation and MOF. 17
2.3. Reperfusion‐hypoxia syndrome
Hypoxia and shock induced by acute respiratory distress syndrome, systemic inflammatory response syndrome, and multiple organ failure may cause hepatic hypoperfusion and dysfunction secondary to hypoxia reperfusion. The reduction in oxygen levels (and the consequent lipid accumulation) during shock and other hypoxic conditions, could lead to hepatocyte necrosis. Overall, the damage that occurs, the increase in reactive oxygen species and the products generated during the reactions can activate some transcription factors and act as second messengers, further increasing the release of pro‐inflammatory cytokines. 4 , 8 , 18 It is well known that hypoxia is a relevant factor involved in liver damage from COVID‐19.
3. DRUG‐INDUCED LIVER INJURY IN COVID‐19
Liver injury during SARS‐CoV‐2 infection may also be related to drug hepatotoxicity, especially 7‐10 days after disease onset, which explains the great variability observed in the different cohort studies in the literature, due to the different drugs used to manage COVID‐19. Treatment of infection involves the use of multiple medications such as anti‐pyretic, anti‐microbial, anti‐viral and anti‐fungal drugs and steroids, which have all been seen to be potential causes of liver damage, although there is still no strong evidence to support this. 19 Consequently, in patients with COVID‐19 there has been an increased incidence of liver dysfunction not only directly related to the action of the virus, but also to the side effects of the drugs used in its management 2 (see Table 2).
TABLE 2.
Type of DILI and mechanism of damage of different approved drugs used for therapy of COVID‐19, adapted from Liver Tox
| Drugs | Type of DILI | Mechanism of liver injury |
|---|---|---|
| Chloroquine | Hepatocellular | Hypersensitivity, idiosyncratic |
| Azithromycin |
Cholestatic hepatitis (more frequent) Hepatocellular |
Hypersensitivity, idiosyncratic |
| Acetaminophen | Hepatocellular | Dose‐dependent |
| Lopinavir/ritonavir | Hepatocellular, cholestatic or mixed | Due to liver metabolism by the cytochrome P450 system (CYP3A4), which may result in the production of a toxic intermediate |
| Tocilizumab | Hepatocellular, predominantly cholestatic | Linked to effects on the immune system or on the IL‐6 pathway, which is important in liver regeneration |
| Remdesivir | Hepatocellular | Idiosyncratic |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Among the drugs initially licensed for use against COVID‐19 was chloroquine; in vitro studies have shown its important activity in the inhibition of viral replication and also a certain effectiveness in vivo. 20 Chloroquine acts either by stopping the cytokine storm or by blocking the activation of CD8+ cells. 21 However, its use has been linked in the past to the onset of fulminating hepatitis. 22 In combination with chloroquine, azithromycin, a broad‐spectrum macrolide, has been used due to its presumable anti‐inflammatory activity, but it is a common cause of hepatotoxicity (DILI) after about 1‐3 weeks of treatment. 23 A retrospective cohort study of 134 patients showed that treatment with chloroquine/azithromycin led to a clinical improvement in 26.8% of patients and although ICU transfer rate and mechanical ventilation, as well as mortality at day 28 were higher in the treatment group compared to controls (p 0.03), no differences in LFTs were found between the two groups. 24 In a randomised controlled multicenter trial, 25 which included 667 patients treated with hydroxychloroquine alone or in combination with azithromycin versus standard treatment, QT interval prolongation and LFTs alterations were shown to be more frequent in treated than in untreated patients. Therefore, among hospitalised patients, the use of hydroxychloroquine, alone or with azithromycin, does not improve the clinical condition after 15 days of treatment, compared to standard therapy, but could instead be responsible for the appearance of adverse events such as DILI or QT interval prolongation.
Most COVID‐19 patients with a very high fever have been treated with anti‐pyretic agents containing paracetamol, one of the drugs mainly responsible for dose‐dependent liver damage and acute liver failure. In particular, patients presenting with NAFLD are more susceptible to liver injury from acetaminophen at lower doses than the healthy population. For this reason, the US Food and Drug Administration suggested lowering the daily paracetamol dosage normally used in patients with pre‐existing liver damage (from 4g to 3g) and the maximum single dosage from 1g to 0.65 g. 26 Unfortunately, this recommendation has not been accepted globally, and patients with COVID‐19 and metabolic syndrome, have been continuously exposed to the risk of severe hepatotoxicity.
The liver is involved in the metabolism of most drugs, including nucleoside analogues and protease inhibitors, also currently being tested in the management of COVID‐19. An increase in aminotransferases, if elevated up to 4‐5 X ULN, could be a sign of hepatotoxicity triggered by these drugs. A recent study reported that the use of lopinavir/ritonavir, as an antiviral treatment against SARS‐CoV‐2 infection, was associated with an alteration of LFTs and an increase in bilirubin serum levels in patients with severe disease. 27 In another series of cases, 55.4% of patients apparently reported severe liver damage after treatment with lopinavir/ritonavir. 28 Fan et al published a retrospective study in which they observed that the use of this drug combination was significantly higher in patients with abnormal LFT than in patients without LFT changes (56.1% vs. 25%, P < 0.009). In this study, 47.3% of discharged patients had elevated LFTs at baseline and 23.7% developed abnormalities during hospitalisation, suggesting that the emerging liver injury was likely due to medication rather than infection. 7 Moreover alterations in LFTs were associated with a long duration of hospitalisation. It is well documented that patients with viral infections (hepatitis C or HIV virus) are prone to developing drug‐induced liver damage (DILI), 29 particularly following the use of antiretroviral therapy, and more specifically HAART therapy. 30 It is not known, whether SARS‐CoV‐2 infection also predisposes to DILI.
Another therapeutic tool in the treatment of COVID‐19 is tocilizumab—a humanised anti‐IL‐6 receptor monoclonal antibody used in patients with severe lung damage and high blood levels of IL‐6. 31 During clinical trials, a slight increase in LFTs was observed, but it was transient and resolved within 2‐6 weeks after commencement of treatment. 32 Several trials are currently under way, aimed at reducing ICU admissions. However, the safety of this therapy must be carefully monitored, as immunosuppression could lead to an increase in the rate of bacterial infections, adversely affecting the clinical condition of patients, especially those in ICU. The use of these immunosuppressive therapies in other diseases occasionally causes fulminant liver failure due to the reactivation of HBV infection in chronic carriers. 32
Remdesivir is an antiviral inhibitor of RNA polymerase, recently approved in the treatment of patients with severe pneumonia from severe COVID‐19. Liver toxicity results have been contradictory, however, and it seems that remdesivir can lead to renal failure or liver dysfunction. 33 , 34 Zampino et al described five cases of patients treated with remdesivir with raised AST/ALT levels, suggesting hepatocellular injury, but without liver failure. 35 However, DILI in Covid‐19 hospitalised patients was of 25.4%, as recently reported in a meta‐analysis. 36
Convalescent plasma is frequently administered to critically ill patients with Covid‐19 and has been reported to improve clinical outcomes. Given the observational data, coming from case series, and those reported from randomised controlled trial, it seems that no significant differences were observed in clinical status or overall mortality between patients treated with convalescent plasma and those receiving placebo. 37 Infusion‐related adverse events were slightly more common in the convalescent plasma group (4.8%; 11 out of 228 patients) than in the placebo group (1.9%; 2 out of 105 patients) (OR 2.62; 95% CI, 0.57‐12.04). Five patients in the convalescent plasma group and none in the placebo group had non‐haemolytic febrile reactions. Increasing of LFTs of any grade have been reported in the convalescent plasma group as 9.6% (22 out of 228) vs 7.6% (7 out of 109). Thus, no significant differences were found in the overall incidence of adverse events (OR 1.21; 95% CI, 0.74 −1.95). However, multiple uncertainties exist regarding to efficacy, appropriate donor selection and neutralizing antibodies titres. 38
Data regarding clinical trials with monoclonal antibodies, involving patients with Covid‐19, have been reported by two groups of researchers. In the first study, Weinreich et al, 39 reported results related to the study of a combination of two monoclonal antibodies, casirivimab and imdevimab (REGN‐COV2), directed against the SARS‐CoV‐2 spike protein. The study enrolled outpatients, within 7 days of symptom onset and within 72 hours of a positive nasopharyngeal swab. Both doses of REGN‐COV2 were associated with few, mainly low‐grade, side effects, and none showed increasing of LFTs. These results complement those obtained by Chen et al, 40 which evaluated three doses of a single monoclonal antibody, bamlanivimab, administered to outpatients presenting with Covid‐19 within 4 days of the onset of symptoms. Reductions in viral RNA levels were detected after 3 days of treatment in all groups; only 14 patients were hospitalised: 5 (1.6%) in the bamlanivimab group and 9 (6.3%) in the placebo. No serious adverse events were registered; the percentage of patients who experienced an adverse event during treatment was 22.3% in the treatment group and 24.5% in placebo group. Diarrhoea, vomiting and nausea were adverse events commonly described in a percentage of cases lower than 5% in both the groups. Infusion‐related reactions were mild and reported in 2.3% of the treatment group as compared with 1.4% in the placebo group. Alteration of LFTs was not reported.
These exciting results suggest that monoclonal antibodies acting as an antiviral agent useful for reducing viral load, seems to be fundamental for the treatment of early Covid‐19, as to avoid hospitalisations and overload for the health system. Many other trials are still ongoing in qualified nursing facilities and among family contacts of patients with SARS‐CoV‐2 infection. However, till now, alteration of LFTs and liver injury in patients treated with both those monoclonal antibodies have never been reported.
4. PRE‐EXISTING LIVER DISEASE DURING COVID‐19
The relationship between pre‐existing liver disease and COVID‐19 has not been carefully studied. It is unclear if chronic liver disease should be considered a risk factor. However, it is important for clinicians to understand the importance of proper clinical monitoring via LFTs during hospitalisation, especially in patients with pre‐existing liver damage, as viral hepatitis and/or NAFLD. Particular attention should also be paid to patients with liver cirrhosis and/or cancer, as their systemic immune impairment status makes these patients more susceptible to SARS‐CoV‐2 infection and therefore requires greater clinical care, through strictly personalised therapeutic protocols.
4.1. COVID‐19 and NAFLD
The acute Covid‐19 pandemic is superimposed on a much slower but equally widespread epidemic, namely metabolic diseases, as obesity and diabetes, resulting from the growing spread of an inadequate lifestyle characterised by excessive consumption of calories and scarce, if not absent, physical activity. This metabolic condition, predispose to a more severe Covid‐19 disease, with greater morbidity and mortality. Diabetes and obesity accentuate the clinical response to SARS‐COV‐2 infection, trigger an altered immune response, which is associated with an atherothrombotic state, and finally maintain an active chronic systemic inflammatory status responsible for severe complications, as septic shock, ARDS and multi‐organ failure. The forced lockdown also contributed to reducing the possibility of physical activity, and at the same time, determining an increase in nutritional intake. Furthermore, the reduced exposure to the sunlight entails a decrease in vitamin D levels, and thus a reduction in anti‐inflammatory activity and consequently an enhancement of insulin resistance, which is the main player of the metabolic syndrome. 41 NAFLD represents the hepatic component of the metabolic syndrome. This condition affects almost one quarter of the Western population, mostly obese, and to date there is no approved drug therapy. Although hepatic steatosis is a benign disease, about 20%‐30% of patients develop necro‐inflammation and hepatic fibrosis (non‐alcoholic steatohepatitis‐NASH) with the possibility of evolving towards cirrhosis. Due to the lack of effective therapies and the spread of obesity, post‐NASH liver cirrhosis is currently the leading cause of liver transplantation. Recently, an international group of experts raised the question of the lack of consistency of the term NAFLD and proposed that of Metabolic Associated Fatty Liver Disease, as MAFLD. 42
The increased incidence of NAFLD poses the risk of a large percentage of the population developing severe complications from COVID‐19. 43 A high concentration of ACE2 receptors has been observed in the small intestine epithelium, which explains the presence of symptoms such as abdominal pain and diarrhoea in infected subjects. The virus then reaches the liver by means of the portal circulation. 44 The liver contains a high number of resident macrophages, Kupffer cells, which could play a crucial role in liver damage following the activation and release of pro‐inflammatory cytokines. It should also be noted that NAFLD promotes the activity of M1 macrophages, which produce pro‐inflammatory cytokines, and suppresses the activity of M2 macrophages, thus contributing to the progression of damage from COVID‐19. 45 Understanding the role of NAFLD in COVID‐19 disease could have important therapeutic and clinical implications. Therefore, patients with COVID‐19 with pre‐existing liver diseases such as NAFLD, and even more so if they are male and in advanced age, should be closely monitored and strongly supported with heparin at a suitable therapeutic dose.
4.2. COVID‐19 and alcoholic liver disease
Social distancing needed to fight the pandemic, and stress, isolation and depression caused by the lockdown resulted in a significant increase in alcohol consumption and related diseases (ALD). Alcoholic liver disease is often associated with diabetes and chronic renal failure, both comorbidities that increase the risk of severe Covid‐19. In particular, in patients with alcoholic steatohepatitis, the use of corticosteroids could lead to an immunosuppressive effect that would further increase the risk of having a more severe Covid‐19. 46
To date, no specific guidelines are available regarding the management of alcoholic hepatitis during COVID‐19, and existing studies recommend caution about steroids administration in patients infected with SARS‐CoV‐2. 47 The pandemic has also raised walls about patients with ALD being considered for liver transplantation, resulting in both increased risk for relapse and difficulties in maintaining linkage to care. Initial observations that the use of non‐steroidal anti‐inflammatory drugs (NSAIDs) worsened the COVID‐19 disease, led to the increasing use of paracetamol, and even if this type of indication is no longer currently accepted, this has led to a rising in hepatotoxicity due to the combined use of paracetamol and alcohol. 48 , 49
4.3. COVID‐19 and viral hepatitis (HBV/HCV)
In patients with chronic hepatitis B in immunotolerant phases with viral suppression under long‐term treatment with nucleos(t)ide analogues, evidence of persistent liver injury and active viral replication after co‐infection with SARS‐CoV‐2 needs to be further investigated. 50
Various studies have shown that chronic viral hepatitis does not appear to increase the risk of a severe course of COVID‐19 or extend the duration of hospitalisation. 51 , 52
COVID‐19 patients co‐infected with chronic HBV could risk hepatitis B reactivation. It is therefore necessary to monitor liver function as well as HBV‐DNA levels, 52 Zou et al studied four patients with chronic HBV‐infection, who were finally diagnosed as ACLF due to a rapid deterioration in function after SARS‐CoV‐2 co‐infection with, progressively, jaundice, coagulation dysfunction and ascites. Liver injury in patients with SARS‐CoV‐2 and chronic HBV co‐infection has been associated with severity and poor prognosis of disease. 53
A recently published retrospective study on the incidence of SARS‐CoV‐2 infection in HCV‐infected patients highlights how the rate of positivity is extremely low. SARS‐COV‐2 infection appears to mainly affect obese and diabetic individuals; however, the degree of hepatic fibrosis does not appear to correlate with an increased risk of infection, either in patients with active HCV infection or in those already treated and who achieved eradication. 54
Despite the rearrangements made within hospitals during lockdown, and the consequent reduction in outpatient visits, the commencement of antiviral therapies for HCV and HBV should be promoted, especially in patients with more advanced liver disease and comorbidities.
4.4. COVID‐19 and liver diseases requiring immunosuppression
In general, patients treated with immunosuppressants are considered to be at greater risk of serious complications from COVID‐19. However, no data suggest that in these patients there is an increased susceptibility to infection. Patients with autoimmune hepatitis (AIH), primitive biliary cholangitis (PBC), overlap syndromes and primitive sclerosing cholangitis have a disrupted immune response that may be relevant in SARS‐CoV‐2 infection. Since ACE2 receptors are expressed in cholangiocytes, infection with SARS‐CoV‐2 could aggravate cholestasis in patients with primary biliary cholangitis. In patients with autoimmune hepatitis and COVID‐19, the effects of glucocorticoid administration on the liver disease are unclear. Immunosuppression may prolong the infectious state but reducing the dose or interrupting the immunosuppressant may cause a flare‐up in a patient with AIH. In patients with a new onset of liver disease, diagnosis and treatment should not be delayed. The APASL and AASLD guidelines 47 suggest maintaining the usual dose for patients on immune‐suppressants without infection. By contrast, in COVID‐19 patients it is recommended to reduce the corticosteroid to a dose that avoids the onset of adrenal insufficiency, while reducing the dosages of azathioprine, mycophenolate mofetil or calcineurin inhibitor should be considered, especially in a context of lymphoma or worsening pneumonia.
4.5. COVID‐19 and cirrhosis /liver cancer
Patients with cirrhosis or liver cancer could be more prone to developing a SARS‐CoV‐2 infection and to a more severe course due to their systemic immunocompromised status. However, there are no data regarding severity, mortality and incidence of complications (infections, hepatic encephalopathy, bleeding from varices, liver failure). 47 An Italian study showed that SARS‐CoV‐2 infection was associated with liver function deterioration and elevated mortality in patients with cirrhosis, whether compensated or decompensated. The main causes of death were respiratory complications but also the sudden worsening of liver function. 55 Further studies should focus on the effect of COVID‐19 on existing liver comorbidities and treatment outcomes. In cirrhotic patients, it is essential to continue follow‐up to avoid worsening the underlying chronic liver disease or underestimating a liver cancer.
4.6. COVID‐19 on Liver Transplantation (LT)
Covid‐19 in LT recipients is unfortunately responsible for a greater number of cases who are hospitalised in intensive care unit (ICU) due to severe pneumonia requiring mechanical ventilation. These patients also have a higher risk of liver damage, evident from alteration of the LFTs, which depends not only on the state of immune‐suppression related to the transplant, but also on comorbidities, such as diabetes, cardiovascular and renal diseases, and the considerable risk of severe drug‐drug interactions. One of the few recent studies that analysed this clinical setting highlighted how age, Hispanic race, metabolic syndrome, the use of antibiotics and vasopressor were able to predict the onset of liver damage, and also its multifactorial nature. 56 Furthermore, liver injury was independently associated with increased mortality. Thus, following LFTs in a LT recipient with Covid‐19, as a warning of liver damage, could help to identify patients at risk for a poor outcome. 57
5. MANAGEMENT OF LIVER PATIENTS WITH COVID‐19
In patients with a mild COVID‐19, liver injuries are often transient and self‐limiting. 58 Average levels of AST, ALT and serum bilirubin are significantly higher in severe or critical cases, 59 such as a reduction in serum albumin. 60 According to recent studies, there is no specific treatment for liver injury during COVID‐19, although in patients with severe liver damage it is advisable to administer medications with a hepatic‐protective effect. 50 The presence of altered liver enzymes should not be considered a contraindication to the use of experimental or off‐label therapies for COVID‐19. However, high transaminase levels (five times the baseline value) exclude patients from experimental treatment. In particular, patients treated with remdesevir, ritonar and tocilizumab should be regularly monitored via LFTs, as these drugs have a documented hepatotoxicity. 47 It appears that a greater severity of SARS‐CoV‐2 infection and advanced age, together with poly‐therapy, predisposes patients to a more severe liver dysfunction. The increase in transaminases, in particular AST, is more common in adult males than in female patients. Therefore, COVID‐19 patients who have pre‐existing liver diseases should be adequately monitored and drug‐to‐drug interactions should be checked before starting COVID‐19 treatments, including antiviral agents, especially in patients with advanced chronic liver. A useful checker tool is available at: https://www.COVID19‐druginteractions.org/checker
6. CONCLUSIONS
The underlying mechanisms of liver injury during SARS‐CoV‐2 remain unclear. Among the most probable hypotheses are viral cytopathic effects, immune imbalance and cytokine storm, ischaemia and/or hypoxia‐reperfusion injury and, finally DILI. A recent meta‐analysis reported that incidence of DILI in Covid‐19 hospitalised patients was of 25%. From a clinical point of view, great care should be taken when monitoring patients to prevent the onset of liver injury and avoid the administration of hepatotoxic drugs. Some studies have reported an increased incidence of acute kidney injury after COVID‐19, possibly due to the presence of SARS‐CoV‐2‐induced inflammation. Damage to the liver and kidneys can affect expectations regarding the metabolism, excretion, dose and concentration of drugs, thus making it difficult to establish the correct therapeutic dose of the drugs without increasing the risk of toxicity. 61 , 62
Continuing studies on severity, mortality and the incidence of complications should be performed in order to understand how pre‐existing liver conditions may influence and worsen the progression of liver disease in patients with SARS‐CoV‐2 infection.
CONFLICT OF INTEREST
None to declare.
AUTHOR CONTRIBUTIONS
Anna Licata, Maria Giovanna Minissale, Marco Distefano and Giuseppe Montalto contributed to concept and study design, helped in drafting the article, collected and analysed data. All authors approved the final version of the manuscript.
ETHICAL STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required as this is a review article with no original research data.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/ygh2.455.
ACKNOWLEDGEMENTS
Guarantor of the article: Anna Licata.
Licata A, Minissale MG, Distefano M, Montalto G. Liver injury, SARS‐COV‐2 infection and COVID‐19: What physicians should really know? GastroHep. 2021;3:121–130. 10.1002/ygh2.455
REFERENCES
- 1. Guan W‐J, Ni Z‐Y, Hu YU, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cai Q, Huang D, Yu H, et al. COVID‐19: abnormal liver function tests. J Hepatol. 2020;73:566‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang Y, Zheng L, Liu L, et al. Liver impairment in COVID‐19 patients: a retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40(9):2095‐2103. [DOI] [PubMed] [Google Scholar]
- 5. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420‐422. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS‐CoV‐2 infected patients. EBioMedicine. 2020;55:102763. 10.1016/j.ebiom.2020.102763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fan Z, Chen L, Li J, et al. Clinical features of COVID‐19‐related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18(7):1561‐1566. 10.1016/j.cgh.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425‐434. 10.1016/S1473-3099(20)30086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu X‐W, Wu X‐X, Jiang X‐G, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS‐Cov‐2) outside of Wuhan, China: retrospective case series. BMJ. 2020;19(368):m606. 10.1136/bmj.m606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adams DH, Hubscher SG. Systemic viral infections and collateral damage in the liver. Am J Pathol. 2006;168:1057‐1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019‐nCoV infection. bioRxiv. 2020. 10.1101/2020.02.03.931766. [DOI] [Google Scholar]
- 14. Gurwitz D. Angiotensin receptor blockers as tentative SARSCoV‐2 therapeutics. Drug Dev Res. 2020;81(7):777‐781. 10.1002/ddr.21689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao B, Ni C, Gao R, et al. Recapitulation of SARS‐CoV‐2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11(10):771‐775. 10.1007/s13238-020-00718-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jose RJ, Manuel A. COVID‐19 cytokine storm: the interplay between infammation and coagulation. Lancet Respir Med. 2020;8(6):e46‐e47. 10.1016/S2213-2600(20)30216-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang X‐J, Cheng XU, Yan Z‐Z, et al. An ALOX12‐12‐ HETE‐GPR31 signaling axis is a key mediator of hepatic ischemia‐reperfusion injury. Nat Med. 2018;24:73‐83. [DOI] [PubMed] [Google Scholar]
- 19. Gambato M, Burra P. Clinical implications of COVID‐19 in patients with chronic liver disease and liver tumor. Updates Surg. 2020;72:237‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID‐19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72‐73. [DOI] [PubMed] [Google Scholar]
- 21. Hu TY, Frieman M, Wolfram J. Insights from nanomedicine into chloroquine efficacy against COVID‐19. Nat Nanotechnol. 2020;15:247‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Makin AJ, Wendon J, Fitt S. Fulminant hepatic failure secondary to hydroxychloroquine. Gut. 1994;35:569‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martinez MA, Vuppalanchi R, Fontana RJ. Clinical and histologic features of azithromycin‐induced liver injury. Clin Gastroenterol Hepatol. 2015;13:369‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kelly M, O’Connor R, Townsend L, et al. Clinical outcomes and adverse events in patients hospitalised with COVID ‐19, treated with off‐ label hydroxychloroquine and azithromycin. Br J Clin Pharmacol. 2021;87(3):1150–1154. 10.1111/bcp.14482 [DOI] [PubMed] [Google Scholar]
- 25. Cavalcanti A, Zampieri F, Rosa R, et al. Hydroxychloroquine with or without Azithromycin in Mild‐to‐Moderate Covid‐19. N Engl J Med. 2020;383(21):2041–2052. 10.1056/NEJMoa2019014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krenzelok EP, The FDA. Acetaminophen Advisory Committee Meeting ‐ what is the future of acetaminophen in the United States? The perspective of a committee member. Clin Toxicol (Phila). 2009;47:784‐789. [DOI] [PubMed] [Google Scholar]
- 27. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med. 2020;382:1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cai Q, Huang D, Ou P, et al. COVID‐19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75(7):1742‐1752. 10.1111/all.14309 [DOI] [PubMed] [Google Scholar]
- 29. Naidoo K, Hassan‐Moosa R. ,Mlotshwa P et al. High rates of drug‐induced liver injury in people living with HIV coinfected with tuberculosis (TB) irrespective of antiretroviral therapy timing during antituberculosis treatment: results from the starting antiretroviral therapy at three points in TB tri. Clin Infect Dis. 2020;10(70):2675‐2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boeckmans J, Rodrigues RM, Demuyser T, Piérard D, Vanhaecke T, Rogiers V. COVID‐19 and drug‐induced liver injury: a problem of plenty or a petty point? Arch Toxicol. 2020;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen LF, Mo YQ, Jing J, Ma JD, Zheng DH, Dai L. Short‐course tocilizumab increases risk of hepatitis B virus reactivation in patients with rheumatoid arthritis: a prospective clinical observation. Int J Rheum Dis. 2017;20:859‐869. [DOI] [PubMed] [Google Scholar]
- 33. de Wit E, Feldmann F, Cronin J, et al. Prophylactic and therapeutic remdesivir (GS‐5734) treatment in the rhesus macaque model of MERS‐CoV infection. Proc Natl Acad Sci USA. 2020;117:6771‐6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rahimi MM, Jahantabi E, Lotfi B. ,Lotfi B et al. Renal and liver injury following the treatment of COVID‐19 by remdesivir. J Nephropathol. 2021;10(2):1. [Google Scholar]
- 35. Zampino R, Mele F, Florio LL, et al. Liver injury in remdesivir‐treated COVID‐19 patients. Hepatol Int. 2020;14(5):881‐883. 10.1007/s12072-020-10077-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kulkarni AV, Kumar P, Tevethia HV, et al. Systematic review with meta‐analysis: liver manifestations and outcomes in COVID‐19. Aliment Pharmacol Ther. 2020;52(4):584‐599. 10.1111/apt.15916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG. A randomized trial of convalescent plasma in covid‐19 severe pneumonia. New Engl J Med. 2021;384(7):619‐629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Devarasetti PK, Rajasekhar L, Baisya R, Sreejitha KS, Vardhan YK. A review of COVID‐19 convalescent plasma use in COVID‐19 with focus on proof of efficacy. Immunol Res. 2021;69(1):18‐25. 10.1007/s12026-020-09169-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN‐COV2, a neutralizing antibody cocktail, in outpatients with covid‐19. New Engl J Med. 2021;384(3):238‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen P, Nirula A, Heller B, et al. SARS‐CoV‐2 neutralizing antibody LY‐CoV555 in outpatients with Covid‐19. New Engl J Med. 2021;384(3):229‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Holly JMP, Biernacka K, Maskell N, Perks CM. Obesity, diabetes and COVID‐19: an infectious disease spreading from the east collides with the consequences of an unhealthy western lifestyle. Front Endocrinol. 2020;17(11):582870. 10.3389/fendo.2020.582870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eslam M, Sanyal AJ, George J, International Consensus Panel . MAFLD: a consensus‐driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(1999–2014): 10.1053/j.gastro.2019.11.312) [DOI] [PubMed] [Google Scholar]
- 43. Ji D, Qin E, Xu J, et al. Non‐alcoholic fatty liver diseases in patients with COVID‐19: a retrospective study. J Hepatol. 2020;73(2):451‐453. 10.1016/j.jhep.2020.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Feng G, Zheng KI, Yan Q‐Q, et al. COVID‐19 and liver dysfunction: current insights and emergent therapeutic strategies. J Clin Transl Hepatol. 2020;8:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lefere S, Tacke F. Macrophages in obesity and non‐alcoholic fatty liver disease: crosstalk with metabolism. JHEP Rep. 2019;1:30‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Da BL, Im GY, Schiano TD. Coronavirus disease 2019 hangover: a rising tide of alcohol use disorder and alcohol‐associated liver disease. Hepatology. 2020;72(3):1102‐1108. 10.1002/hep.31307 [DOI] [PubMed] [Google Scholar]
- 47. Fix OK, Hameed B, Fontana RJ, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID‐19 pandemic: AASLD expert panel consensus statement. Hepatology. 2020;72(1):287‐304. 10.1002/hep.31281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Day M. Covid‐19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;17(368):m1086. 10.1136/bmj.m [DOI] [PubMed] [Google Scholar]
- 49. Torjesen I. Covid‐19: ibuprofen can be used for symptoms, says UK agency, but reasons for change in advice are unclear. BMJ. 2020;17(369):m1555. 10.1136/bmj.m1555 [DOI] [PubMed] [Google Scholar]
- 50. Zhang C, Shi L, Wang FS. Liver injury in COVID‐19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen L, Huang S, Yang J, et al. Clinical characteristics in patients with SARS‐CoV‐2/HBV co‐infection. J Viral Hepatitis. 2020;27:1504‐1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu J, Wang T, Cai Q, et al. Longitudinal changes of liver function and hepatitis B reactivation in COVID‐19 patients with pre‐existing chronic hepatitis B virus infection. Hepatol Res. 2020;50:1211‐1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zou X, Fang M, Li S, et al. Characteristics of liver function in patients with SARS‐CoV‐2 and chronic HBV coinfection. Clin Gastroenterol Hepatol. 2020;19(3):597‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Butt AA, Yan P. Rates and characteristics of SARS‐CoV‐2 infection in persons with hepatitis C virus infection. Liver Int. 2021;41(1):76‐80. 10.1111/liv.14681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Iavarone M, D'Ambrosio R, Soria A, et al. High rates of 30‐day mortality in patients with cirrhosis and COVID‐19. J Hepatol. 2020;73(5):1063‐1071. 10.1016/j.jhep.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rabiee A, Sadowski B, Adeniji N, Perumalswami PV, Nguyen V. Liver injury in liver transplant recipients with coronavirus disease 2019 (COVID‐19): U.S. multicenter experience. Hepatology. 2020;72(6):1900‐1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jayant K, Reccia I, Virdis F, et al. COVID‐19 in hospitalized liver transplant recipients: an early systematic review and meta‐analysis. Clin Transplant. 2021:e14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bangash MN, Patel J, Parekh D. COVID‐19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu C, Jiang ZC, Shao CX, et al. Preliminary study of the relationship between novel coronavirus pneumonia and liver function damage: a multicenter study. Zhonghua Gan Zang Bing Za Zhi. 2020;28:148‐152. [DOI] [PubMed] [Google Scholar]
- 60. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Alqahtani SA, Schattenberg JM. Liver injury in COVID‐19: the current evidence. United Eur Gastroenterol J. 2020;8(5):509‐519. 10.1177/2050640620924157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pan X‐W, Xu DA, Zhang H, et al. Identification of a potential mechanism of acute kidney injury during the COVID‐19 outbreak: a study based on single‐cell transcriptome analysis. Intensive Care Med. 2020;46(6):1114‐1116. 10.1007/s00134-020-06026-1 [DOI] [PMC free article] [PubMed] [Google Scholar]


