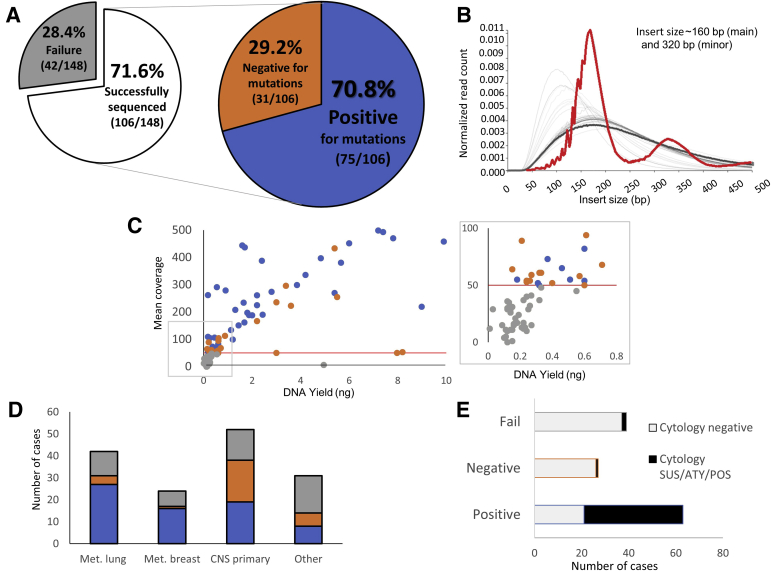
Figure 2.
Memorial Sloan Kettering Integrated Mutation Profiling of Actionable Cancer Targets assay performance. A: Sequencing success rate was 71.6% (106/148 cases with quantifiable input DNA), whereas failure rate was 28.4% (42/148, no variants detected, coverage <50×). Somatic alterations were observed in cell-free DNA (cfDNA) in 70.8% (positive, 75/106) of successful cases. B: Insert size distribution of simultaneously sequenced samples shows that the single cerebrospinal fluid (CSF) cfDNA sample (highlighted in red) demonstrates insert sizes at approximately 160 bp (main) and 320 bp (minor), consistent with previously reported results for other cfDNA specimens, distinguishing them from formalin-fixed, paraffin-embedded tumor samples (light gray) and paired normal blood (dark gray). C: Mean coverage versus sample DNA yield (positive, blue; negative, orange; fail, gray); although failure rates were higher among low-concentration cases (less than approximately 0.3 ng), adequate coverage (>50×; red line) and positive results could still be obtained for some samples, even at low cfDNA levels (inset). D: CSF cfDNA results by tumor type (positive, blue; negative, orange; fail, gray). E: CSF cytology correlations with cfDNA results. ATY, atypical; CNS, central nervous system; Met., metastatic; POS, positive for malignant cells; SUS, suspicious.