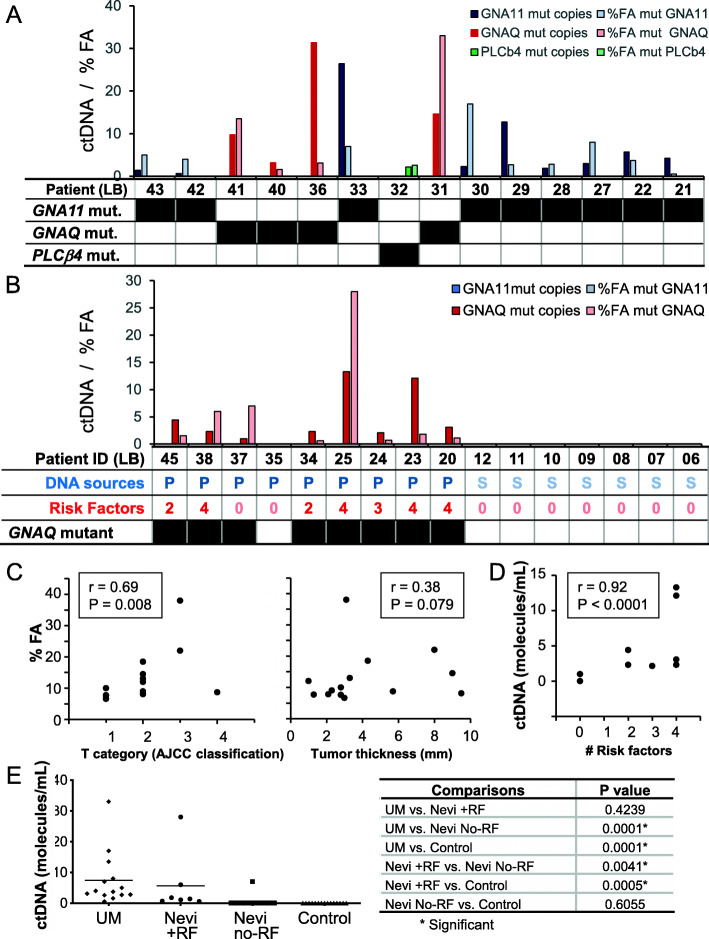
Fig. 4.
The levels of mutated ctDNA in UM patients and patients with uveal nevi correlated with the stage of disease progression and with the presence of risk factors for malignant transformation, respectively. A. UM patients: levels of GNA11, GNAQ and PLCβ4 mutated ctDNA (molecules/mL of plasma) are shown in dark blue, red and green, respectively, and %FA of GNA11, GNAQ and PLCβ4 is shown in light color (the table shows the mutation status in the analyzed loci). B. Nevi: levels of ctDNA molecules/mL of plasma (dark) and %FA (in light color) in patients (the table shows the mutation status in the analyzed loci). Note that in all patients, only a GNAQ mutation was detected. P:plasma. S:serum. C. Left panel: the %FA obtained with the UM samples were plotted against the T category of the AJCC staging, which displayed a significant positive correlation (r = 0.69, P = 0.008). Right panel: The %FA obtained with the UM samples were plotted against tumor thickness (mm); no correlation was found (r = 0.38, P = 0.079). D. The levels of mutated ctDNA in plasma samples obtained from patients with nevi were plotted against the number of risk factors in every patients, which displayed a significant positive correlation (r = 0.92, P < 0.0001). E Left panel: Scatter plot depicting the levels of mutated ctDNA in the plasma of UM patients and patients with nevi displaying or not risk factors for malignant transformation, and serum of healthy blood donors. Note the increased levels of ctDNA that accompany increased risk factors. The table (on the right) depicts the comparisons used and the levels of significance for differences (P value)