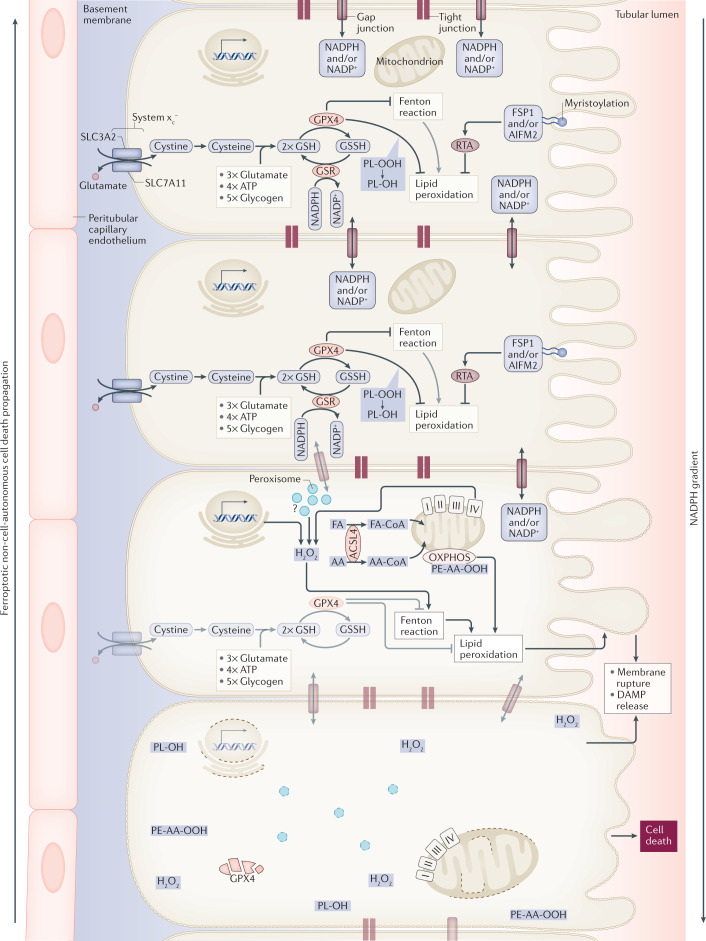
Fig. 2. Synchronized regulated necrosis.
In most cases, it is currently unclear how a necrotic zone of dead cells propagates during an event of sepsis or ischaemia. Based on time-lapse videos obtained from isolated perfused renal tubules that underwent ferroptosis, the concept of synchronized regulated necrosis emerged96. Although this phenomenon has not been observed in humans, necrotic tubules in the urine of patients and experimental intravital microscopy videos suggest that cell death occurs in a wave of death97,222. Given the connections between cells in a functional syncytium, such as a renal tubule, the adrenal gland or the myocardium, it is tempting to speculate that the intracellular redox capacity (NADPH concentration) diffuses through intercellular junctions. If the redox capacity decreases in a dying cell, an NADPH gradient forms, which leaves the closest neighbour at high risk of undergoing ferroptosis. At least from heart attacks and insults of acute kidney injury, it is now clear that the bulk of the necrotic area that occurs during myocardial infarction or tubular necrosis originates from cells that underwent ferroptosis. We speculate that similar mechanisms might occur in the adrenal gland during sepsis or during resuscitation in intensive care units and potentially in other endocrine organs. AA, arachidonic acid; DAMP, damage-associated molecular pattern; FA, fatty acid; GPX4; glutathione peroxidase 4; GSH, glutathione; GSR, glutathione-disulfide reductase; GSSG, glutathione disulfide; HMGCR, 3-hydroxy-3-methylglutaryl-CoA reductase; PL-OH, phospholipid alcohol; PL-OOH, phospholipid hydroperoxide; RTA, radical-trapping antioxidant.