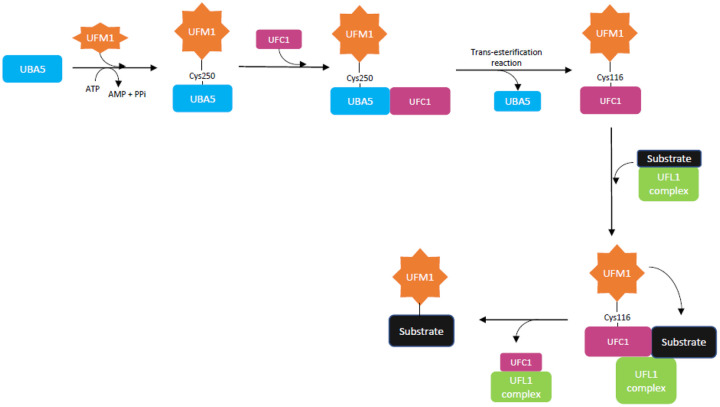
Figure 1.
In the first step of UFMylation, UFM1 is activated by UBA5 (E1-like enzyme) and forms a thioester bond with the Cys250 residue of UBA5. In the second step, UFC1 (E2-like enzyme) binds to UBA5, and UFM1 is transferred to the Cys116 residue of UFC1 by a trans-esterification reaction. In the third step, the UFL1 complex (E3-like enzyme) brings a substrate to the UFC1–UFM1 complex, and UFM1 is covalently conjugated to the substrate. The functional studies performed in this study measure the presence of the UFM1–UBA5 and UFM1–UFC1 conjugates.