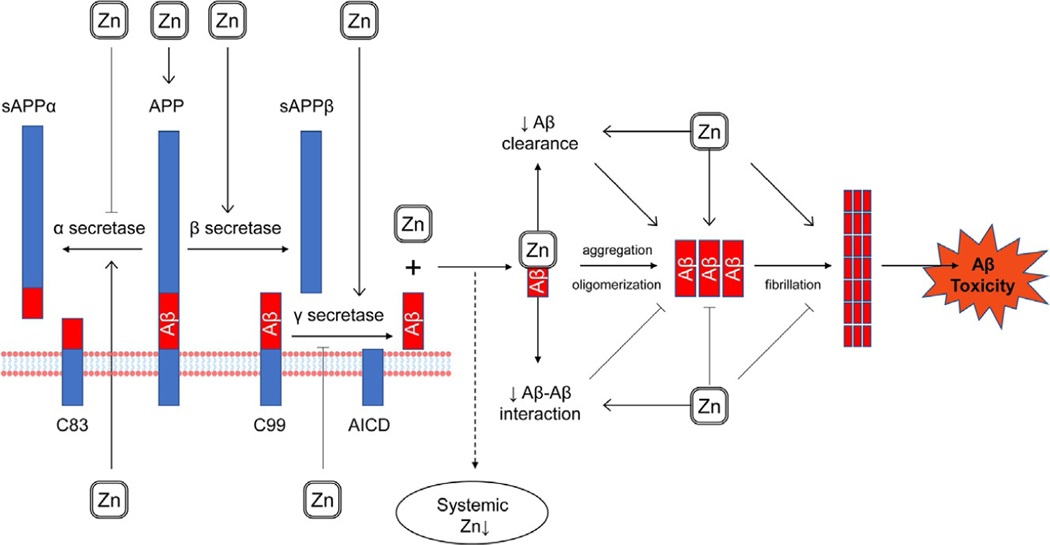
Fig. 2.
The impact of Zn2+ on amyloid production and processing. Zn2+ is capable of direct interaction with amyloid β, although data on this interaction have yet to be fully delineated. On one hand, Zn2+-Aβ binding induced protein oligomerization (Istrate et al., 2016) and subsequent aggregation (Khatua, Mondal, & Bandyopadhyay, 2019), whereas its prevention was shown to reduce Aβ toxicity (Takeda & Tamano, 2015). On another hand, certain studies reported that Zn2+ may decrease Aβ-Aβ interactions (Hane, Hayes, Lee, & Leonenko, 2016) and reduced fibril elongation (Abelein, Gräslund, & Danielsson, 2015). In turn, direct interaction between Zn2 + and Aβ results in plaque Zn sequestration ultimately leading to a decrease in systemic Zn levels (Baum et al., 2010). In addition to direct interaction with Aβ, Zn2+ was also shown to modulate amyloidogenic pathways (Kim, Lim, & Kim, 2018), although the data are highly contradictory. Particularly, certain studies demonstrated antiamyloidogenic effects of Zn2+ through inhibition of γ-secretase (Li, Liu, Xu, Li, & Xu, 2018), whereas others revealed activation of amyloidogenic pathway through inhibition of α-secretase and a concomitant activation of β- and γ-secretase (Wang et al., 2010).