Abstract
Context
Diets high in plant-based protein have gained popularity due to increasing health concerns regarding consumption of animal products. Though links between intakes of certain protein-rich foods and reproductive disorders have been suggested, the relationship of overall animal and vegetable proteins with reproductive hormones among reproductive-aged women is unknown.
Objective
To evaluate the associations between the intake of dietary protein with reproductive hormones and sporadic anovulation among reproductive-aged women.
Design
A prospective cohort study, 2005–2007.
Setting
University at Buffalo, western New York, United States.
Participants
A total of 259 premenopausal women (18–44 years) without dietary restrictions.
Main Outcome Measure(s)
Serum reproductive hormones were determined up to 8 times per cycle for 2 cycles. Protein intake was assessed the day prior to hormone assessment at 4 visits/cycle using 24-hour recalls.
Results
Overall, 84% of participants met the recommended dietary allowance for total protein set for reproductive-aged women. Neither total nor animal protein intake were associated with reproductive hormones or anovulation. However, vegetable protein intake in the lowest tertile was associated with lower luteal phase progesterone (-18.0%, 95% confidence interval [CI] -30.2, -3.6), higher follicle-stimulating hormone (3.8%, 95% CI 0.2, 7.6), and a higher risk of anovulation (risk ratio [RR] 2.53, 95% CI 1.21, 5.26), compared with the middle tertile. Nuts and seeds were the only protein-rich foods associated with an elevated risk of anovulation (RR 2.12, 95% CI 1.17, 3.85).
Conclusions
Findings suggest that among women who meet the recommended dietary allowance for total protein, low intake of vegetable, but not animal, protein may disturb normal ovulatory function.
Keywords: dietary protein, animal protein, vegetable protein, reproductive hormones, anovulation
Although both animal products and certain vegetables are major sources of dietary protein, obtaining protein mainly from plant-based foods has been gaining popularity. This has been prompted by growing concerns regarding elevated risks of cardiovascular disease (1), diabetes (2), and cancer (3), associated with high consumption of red and processed meat. Exposure to environmental contaminants, such as organic compounds (4), antimicrobial residues (5), and growth hormones (5), through consumption of animal products has further motivated this movement toward plant-based diets. These diets have been suggested as beneficial for planetary health as well (6).
Besides its influence on cardiometabolic health, dietary protein intake has also been linked to reproductive disorders, with a study finding that a high intake of animal products was associated with an increased risk of endometriosis (7). Furthermore, high consumption of animal protein was related to an elevated risk of ovulatory infertility, whereas a protective role of vegetable protein intake was suggested (8). Despite such links with reproductive disorders, the relationship of dietary protein intake with reproductive hormones among healthy women is not well understood. Only a few studies have examined dietary protein in relation with reproductive hormones and ovulation; however, most studies were done among women following vegetarian diets or have only evaluated vegetarian diets as the exposure (9, 10), which limits understanding of the broader role of specific protein sources for reproductive hormones and ovulatory function. More recently, in healthy premenopausal women we found a reduction in serum estradiol concentrations with higher intake of dairy products (11), one of the major sources of dietary protein in the American diet (12). Such observations between specific types of protein-rich food intake and reproductive hormones necessitate an investigation of overall dietary protein intake in relation to changes in reproductive hormones in women of reproductive age, as it may contribute to evidence-based dietary recommendations. As such, the objective of this study was to evaluate relationships of the consumption of total, animal, and vegetable proteins, as well as specific food sources (eg, red meat, poultry, fish, egg, and plant-based protein) with differences in reproductive hormone concentrations and sporadic anovulation among a cohort of healthy premenopausal women.
Subjects and Methods
Design and study population
This was a prospective cohort study of 259 women enrolled in the BioCycle Study (2005–2007). Study participants were healthy and regularly menstruating women with self-reported cycle lengths of 21–35 days for the past 6 months, aged 18–44 years, residing in western New York. Women who were using oral contraceptives and taking vitamin and mineral supplements at baseline, pregnant or breastfeeding in the past 6 months, diagnosed with certain chronic conditions (eg, diabetes mellitus) including a history of menstrual and ovulation disorders and uterine abnormalities (eg, uterine fibroids and polycystic ovary syndrome), or having a self-reported body mass index (BMI) of <18 or >35kg/m2 at screening were not eligible for the study (13). Vegetarians were not included in the study, as women planning to consume a restricted diet during the study period or a diet high in phytoestrogens (eg, soy-based diet) were not eligible for the study.
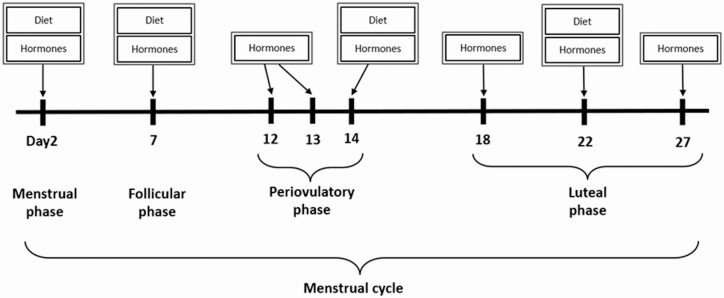
Two-hundred fifty women were followed for 2 menstrual cycles and 9 women were followed for 1 cycle. Participants provided blood samples up to 8 times across the menstrual cycle for hormonal assessment at particular phases of the menstrual cycle (Fig. 1). Specifically, blood samples were collected at the 2nd day of menstruation (1 visit), midfollicular phase (1 visit), periovulatory phase (3 visits), and early, mid-, and late luteal phase (3 visits) in each cycle. Each visit was scheduled according to an algorithm that took each woman’s reported cycle-length history into consideration, along with fertility monitors (Clearblue Easy Fertility Monitor; Inverness Medical, Waltham, MA, USA) used to determine the timing of midcycle visits (14). Overall, 94% of participants completed at least 7 clinic visits per cycle. The University at Buffalo Health Sciences Institutional Review Board (IRB) approved the study and served as the IRB designated by the National Institutes of Health for this study under a reliance agreement. All participants provided written informed consent.
Figure 1.
Collection of blood specimens (8 times) and dietary data (4 times) per menstrual cycle: BioCycle Study, New York, 2005–2007.
Dietary assessment
Participants completed an interviewer-assisted 24-hour dietary recall up to 4 times per cycle for 2 cycles at the study visits corresponding to menstruation, midfollicular phase, expected ovulation, and midluteal phase of the menstrual cycle, capturing dietary intake in the day prior to hormone assessment (Fig. 1). Approximately 87% of the participants completed all 4 dietary recalls per cycle. Dietary intake data were collected and analyzed using the Nutrition Data System for Research software version 2005 developed by the Nutrition Coordinating Center, University of Minnesota (Minneapolis, MN, USA). Intake of total protein, animal protein, and vegetable protein, as well as percent energy from proteins were estimated. Intakes of specific protein foods, including all meats (red meats, poultry, and processed meats), red meats (beef, veal, lamb, pork, game, and organ meats), poultry, processed meats (cured pork, cold cuts, and sausage), fish (fish and shellfish), eggs, meat alternatives (veggie burgers, tofu, tempeh, textured vegetable protein, and soy nuts), legumes (dried beans, mature lima beans, refried beans, and beans in sauce and recipes), nuts and seeds, and nut and seed butters, were also estimated. Intakes of animal proteins were estimated from the sum of consumption of red meats, poultry, processed meats, fish, and eggs. Proteins derived from intakes of all other protein-rich foods, including meat alternatives, legumes, nuts and seeds, and nut and seed butters, were considered vegetable protein.
Reproductive hormone measurement
Reproductive hormones were measured in fasting serum samples collected at each cycle visit (up to 8 visits per cycle for up to 2 cycles). Concentrations of estradiol (pg/mL), follicle-stimulating hormone (FSH, mIU/mL), Luteinizing Hormone (ng/mL), progesterone (ng/mL), and sex hormone-binding globulin (SHBG, nmol/L) were measured by using solid-phase competitive chemiluminescent enzymatic immunoassays by Specialty Laboratories Inc (Valencia, CA, USA) on the DPC Immulite 2000 analyzer (Siemens Medical Solutions Diagnostics, Deerfield, IL, USA) at the Kaleida Health Center for Laboratory Medicine (Buffalo, NY, USA). Concentrations of total testosterone (ng/dL) were determined by liquid chromatography/tandem mass spectrometry employing a Shimadzu Prominence Liquid Chromatography (Shimadzu Scientific Instruments, Inc, Columbia, MD, USA) with an ABSceix 5500 tandem mass spectrometer (AB SCIEX, Framingham, MA, USA) at the University of Minnesota (Minneapolis, MN, USA). Increased sensitivity was obtained by the use of Mobile Phase B (100% acetonitrile) with a low standard of 4ng/dL added to the standard curve. Across the study period the coefficients of variation (CV) for these tests reported by the laboratory were <10% for estradiol and SHBG, <5% for LH and FSH, <14% for progesterone, and <7% for testosterone. Cycles with progesterone concentrations ≤5ng/mL and no observed serum LH peak during the mid- or late luteal phase were considered anovulatory (15). Overall, 42/509 cycles (8.3%) were classified as anovulatory cycles.
Covariate assessment
Participants provided information on demographics (ie, age, race, education level, marital status), lifestyle factors (ie, physical activity, cigarette smoking), and their reproductive health history (ie, parity, past oral contraceptive use, etc.) through questionnaires at baseline. Alcohol consumption was assessed by 24-dietary recall. Physical activity was assessed using the self-administered 2002 long-form International Physical Activity Questionnaire (IPAQ), and categorized into low, moderate, and high based on calculations using the standard IPAQ cutoffs (16). Trained staff measured weight and height of each participant to calculate BMI. All covariates assessed had at least a 94% response rate. Concentrations of heavy metals, including cadmium, mercury, and lead, were quantified in blood samples collected at baseline using inductively coupled plasma mass spectrometry at the Division of Laboratory Sciences of the National Center for Environmental Health at the Centers for Disease Control and Prevention (Atlanta, GA, USA). The CV were 4.3% at concentrations of 2.04 μg/dL for cadmium, 3.2% at 5.77 μg/L for mercury, and 2.6% at 2.89 μg/L for lead.
Statistical analysis
Demographic and lifestyle factors and intake of key nutrients were compared across tertiles of percent energy from animal and vegetable protein intake averaged over the study period, using Fisher’s exact tests and analysis of variance. The intake of total protein in our sample was compared to the recommended dietary allowance (RDA) set for reproductive-aged women (17) and that reported among 19–30 years of age women in the general US population in 2003–2004 (18).
Weighted linear mixed models were used to calculate percent differences and 95% confidence intervals (CIs) between protein tertile (ie, comparing lower to middle tertile [reference] or higher to middle tertile [reference]) and log-transformed serum concentrations of reproductive hormone and metabolic makers. Protein intake was allowed to vary across the cycle, as significant changes in the consumption of protein across the cycle have been observed previously (19). Models for progesterone were restricted to measurements taken during the luteal phase. Models accounted for repeated measures and inverse probability weights were used to account for the time-varying complex feedback mechanisms between hormones during the menstrual cycle (20). Models were adjusted for age (years), race (white, black, or other), BMI (kg/m2), physical activity (low, moderate, or high), alcohol consumption (grams/day), and fiber and total energy intake. Since women could be concurrently exposed to environmental contaminants, such as organic compounds (4), antimicrobial residues, and hormones (5), through consumption of specific protein-rich foods and these contaminants could impact reproductive hormones, models were adjusted for heavy metals (ie, blood cadmium, mercury, and lead) as markers of environmental contaminants. For sporadic anovulation, Poisson regression models with robust error variance were used to estimate risk ratios (RRs) and 95% CIs for the association with average intake of protein before ovulation (eg, consumption of protein in menstrual and follicular phases of each cycle). Models were adjusted for the covariates included in the reproductive hormone models described above.
Macronutrient substitution models were constructed to investigate the impact on either reproductive hormones or anovulation of replacing protein with the same amount of energy from carbohydrate or fat. For example, the carbohydrate substitution model was constructed by additionally adjusting for fat intake. The result can be interpreted as the changes in serum hormones in association with increasing tertile of protein intake in place of carbohydrates, while maintaining constant caloric intake (21). Similarly, the fat substitution model additionally adjusted for carbohydrate intake and the result can be interpreted as the changes in hormone levels in association with increasing tertile protein intake in place of fat. The associations between replacing animal protein with vegetable protein, and vice versa, were also examined by adjusting for energy from other sources (ie, fat, carbohydrate) and another type of protein (ie, animal or vegetable protein).
Relationships between reproductive hormones and anovulation were further assessed by protein-rich food sources (ie, all meats, red meats, poultry, processed meats, fish, eggs, meat alternatives, legumes, nuts and seeds, and nut and seed butters). As described above, weighted linear mixed models and modified Poisson regression models were used for reproductive hormones and anovulation, respectively, and adjusted for fat intake and previously listed covariates. Due to a substantial proportion of participants having zero servings of intake for certain protein foods at each study visit (eg, 65% of visits with 0 serving for red meats and 90% for nuts and seeds), intakes of protein-rich foods were averaged per cycle for hormone analysis. For anovulation analyses, average intake of protein-rich foods before ovulation was used. As a result, intakes of all meats, red meats, poultry, and processed meats were examined continuously (per serving increase) and intakes of fish, eggs, meat alternatives, legumes, nuts and seeds, and nut and seed butters were examined by any versus no intake. SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
Women in the BioCycle study were generally young (mean age: 27.3 years), had a normal BMI on average (mean BMI: 24.1kg/m2), were physically active (35% moderate, 55% high), and were mostly nonsmokers (96.0%) (Table 1). Demographic characteristics did not differ by levels of animal protein intake. However, women with higher vegetable protein intake were more likely to be younger, identify as a race other than white or black, have more than a high school education, be unmarried, and nulliparous. Over the study period, participants obtained, on average, 15.7% of calories from protein, 33.9% from fat, and 50.8% from carbohydrates. Overall, 84% of women consumed more than 46 g of protein per day, the RDA set for reproductive-aged women (17). The daily average intake of total protein was 62.2 g, where approximately two-thirds of which was obtained from animal protein, and this was slightly lower than that reported among 19–30 years of age women in the general US population in 2003–2004 (72.1 g of total protein per day) (18). The most common food source of animal protein was poultry (mean 1.52 servings/day), followed by red meats (mean 0.88 servings/day), processed meats (mean 0.68 servings/day), fish (mean 0.42 servings/day), and eggs (mean 0.30 servings/day). Vegetable protein was most commonly derived from consumption of nuts and seeds (mean 0.20 servings/day), followed by legumes (mean 0.14 servings/day), nut and seed butters (mean 0.13 servings/day), and meat alternatives (mean 0.11 servings/day).
Table 1.
Description of the study cohort by tertiles of average percent energy from animal and vegetable protein intake across the study period
| Average Percent Energy from Animal Protein Intake | Average Percent Energy from Vegetable Protein Intake | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Tertile 1 | Tertile 2 | Tertile 3 | P-valuea | Tertile 1 | Tertile 2 | Tertile 3 | P-valuea | |
| N | n = 259 | 86 | 87 | 86 | 86 | 87 | 86 | ||
| Range: % of total energy | 0.6, 9.0 | 9.0, 11.6 | 11.6, 21.2 | 2.6, 4.7 | 4.7, 5.6 | 5.6, 11.8 | |||
| Demographics | |||||||||
| Age, yrs: mean ± SD | 27.3 ± 8.2 | 27.0 ± 7.9 | 27.6 ± 8.2 | 27.3 ± 8.6 | 0.88 | 27.0 ± 8.4 | 29.1 ± 9.1 | 25.7 ± 6.8 | 0.02 |
| BMI, kg/m2: mean ± SD | 24.1 ± 3.9 | 23.9 ± 4.1 | 23.8 ± 3.5 | 24.5 ± 4.0 | 0.38 | 24.7 ± 4.1 | 24.1 ± 3.4 | 23.5 ± 4.0 | 0.13 |
| Physical activity: n (%) | |||||||||
| Low | 25 (10) | 8 (9) | 11 (13) | 6 (7) | 0.28 | 7 (8) | 5 (6) | 13 (15) | 0.06 |
| Moderate | 92 (35) | 37 (43) | 28 (32) | 27 (31) | 24 (28) | 34 (39) | 34 (40) | ||
| High | 142 (55) | 41 (48) | 48 (55) | 53 (62) | 55 (64) | 48 (55) | 39 (45) | ||
| Race: n (%) | |||||||||
| White | 154 (60) | 54 (63) | 48 (55) | 52 (60) | 0.57 | 45 (52) | 62 (71) | 47 (55) | 0.0006 |
| Black | 51 (20) | 15 (17) | 22 (25) | 14 (16) | 28 (33) | 10 (11) | 13 (15) | ||
| Otherb | 54 (20) | 17 (20) | 17 (20) | 20 (23) | 13 (15) | 15 (17) | 26 (30) | ||
| ≤ High school education: n (%) | 33 (13) | 11 (13) | 12 (14) | 10 (12) | 0.97 | 16 (19) | 12 (14) | 5 (6) | 0.03 |
| Current smoker: n (%) | 10 (4) | 2 (2) | 5 (6) | 3 (3) | 0.62 | 4 (5) | 4 (5) | 2 (2) | 0.78 |
| Married: n (%) | 66 (25) | 20 (23) | 25 (29) | 21 (24) | 0.73 | 20 (23) | 30 (34) | 16 (19) | 0.05 |
| Nulliparousc: n (%) | 187 (72) | 70 (81) | 59 (69) | 58 (71) | 0.14 | 62 (75) | 55 (64) | 70 (83) | 0.02 |
| Past OC Used: n (%) | 140 (54) | 45 (52) | 53 (62) | 42 (51) | 0.29 | 46 (55) | 50 (58) | 44 (52) | 0.71 |
| Dietary Intake: mean ± SD | |||||||||
| % Calories from protein | 15.7 ± 2.9 | 12.9 ± 1.5 | 15.6 ± 1.3 | 18.7 ± 2.3 | <0.0001 | 15.5 ± 2.8 | 15.8 ± 2.6 | 15.9 ± 3.4 | 0.70 |
| % Calories from animal protein | 10.3 ± 3.3 | 6.7 ± 2.1 | 10.3 ± 0.7 | 13.7 ± 2.0 | <0.0001 | 11.5 ± 2.8 | 10.6 ± 2.7 | 8.7 ± 3.8 | <0.0001 |
| % Calories from vegetable protein | 5.4 ± 1.5 | 6.2 ± 1.8 | 5.2 ± 1.2 | 5.0 ± 1.3 | <0.0001 | 4.0 ± 0.5 | 5.2 ± 0.3 | 7.2 ± 1.4 | <0.0001 |
| % Calories from fat | 33.9 ± 5.4 | 32.4 ± 5.5 | 33.5 ± 4.8 | 35.8 ± 5.5 | 0.0001 | 36.8 ± 4.9 | 33.9 ± 4.6 | 31.0 ± 5.2 | <0.0001 |
| % Calories from carbohydrates | 50.8 ± 7.1 | 55.5 ± 6.5 | 51.3 ± 5.3 | 45.7 ± 5.8 | <0.0001 | 47.8 ± 6.4 | 50.4 ± 6.1 | 54.3 ± 7.4 | <0.0001 |
| % Calories from alcohol | 1.0 ± 2.0 | 1.1 ± 2.0 | 0.9 ± 1.6 | 1.0 ± 2.2 | 0.79 | 0.9 ± 1.7 | 1.3 ± 2.3 | 0.9 ± 1.8 | 0.33 |
| Total energy intake, kcal/day | 1607.4 ± 354.3 | 1650.2 ± 385.1 | 1643.8 ± 329.9 | 1528.0 ± 336.3 | 0.04 | 1633.0 ± 347.3 | 1670.4 ± 354.7 | 1518.2 ± 347.1 | 0.01 |
| Total protein, g/day | 62.2 ± 15.6 | 53.2 ± 13.5 | 63.8 ± 13.2 | 69.6 ± 15.5 | <0.0001 | 62.5 ± 15.0 | 65.1 ± 15.4 | 59.0 ± 16.0 | 0.04 |
| Animal protein, g/day | 40.7 ± 14.4 | 28.2 ± 11.2 | 42.8 ± 9.5 | 50.9 ± 12.1 | <0.0001 | 46.0 ± 12.5 | 43.8 ± 12.7 | 32.1 ± 14.2 | <0.0001 |
| Vegetable protein, g/day | 21.4 ± 7.4 | 24.8 ± 8.8 | 20.8 ± 5.6 | 18.6 ± 6.1 | <0.0001 | 16.4 ± 4.4 | 21.0 ± 4.7 | 26.7 ± 8.3 | <0.0001 |
| Fat, g/day | 62.1 ± 18.5 | 61.5 ± 20.5 | 62.9 ± 17.6 | 62.0 ± 17.6 | 0.88 | 68.1 ± 18.6 | 64.4 ± 17.6 | 53.9 ± 16.6 | <0.0001 |
| Carbohydrates, g/day | 201.1 ± 49.0 | 223.9 ± 50.5 | 206.5 ± 40.4 | 172.9 ± 41.4 | <0.0001 | 192.9 ± 45.5 | 207.4 ± 48.8 | 203.0 ± 51.8 | 0.14 |
| Alcohol, g/day | 2.7 ± 5.2 | 3.0 ± 5.2 | 2.4 ± 4.6 | 2.5 ± 5.6 | 0.72 | 2.3 ± 4.0 | 3.5 ± 6.4 | 2.2 ± 4.7 | 0.21 |
| Fiber, g/day | 13.6 ± 5.6 | 15.8 ± 6.9 | 12.8 ± 4.8 | 12.1 ± 3.8 | <0.0001 | 10.2 ± 2.8 | 13.6 ± 3.7 | 16.9 ± 7.0 | <0.0001 |
Abbreviations: BMI, body mass index; OC, oral contraceptives; SD, standard deviation.
a P-values based on analysis of variance and Fisher’s exact test where appropriate.
b Comprised of 1 American Indian/Alaska Native, 13 Asian Indian, 10 Chinese, 2 Filipino, 2 Japanese, 3 Korean, 3 Vietnamese, 6 other Asian, 1 Native Hawaiian, and 13 other race.
c 6 women had missing data on parity.
d 4 women had missing data on past OC use.
Though total and animal protein intakes were largely not associated with reproductive hormones, vegetable protein intake was associated with hormone concentrations (Table 2). Specifically, the lowest tertile of vegetable protein intake was associated with lower luteal phase progesterone (-18.0% difference, 95% CI -30.2, -3.6) and higher FSH levels (3.8% difference, 95% CI 0.2, 7.6) as compared with the middle tertile of intake. In substitution models, increases in protein intake in place of other energy sources, including carbohydrates and fat, did not meaningfully alter associations with reproductive hormones (Table 3).
Table 2.
Weighted linear mixed models evaluating the association between daily protein intakes assessed up to 4 times per menstrual cycle (censtruation, midfollicular phase, expected ovulation, and midluteal phase) for 2 cyclesa and percent difference in log-transformed serum reproductive hormone concentrations measured up to 8 times per menstrual cycle for 2 cycles
| Unadjusted | Adjustedb | |||
|---|---|---|---|---|
| Tertile 1 vs Tertile 2 | Tertile 3 vs Tertile 2 | Tertile 1 vs Tertile 2 | Tertile 3 vs Tertile 2 | |
| % Difference (95% CI) | % Difference (95% CI) | % Difference (95% CI) | % Difference (95% CI) | |
| Total protein (% energy) | ||||
| Estradiol (pg/mL) | 0.4 (-4.5, 5.5) | 1.8 (-3.2, 7.0) | 0.4 (-4.5, 5.5) | 1.5 (-3.5, 6.8) |
| Progesterone (ng/mL)c | -6.0 (-20.5, 11.0) | 1.6 (-13.3, 18.9) | -4.9 (-19.3, 12.1) | -0.2 (-14.7, 16.7) |
| LH (ng/mL) | -0.8 (-5.9, 4.5) | -2.1 (-7.1, 3.3) | -1.0 (-6.2, 4.4) | -2.1 (-7.2, 3.4) |
| FSH (mIU/mL) | 0.4 (-3.0, 4.0) | -0.4 (-3.9, 3.2) | 0.4 (-3.1, 4.0) | -0.5 (-4.0, 3.2) |
| Testosterone (ng/dL) | 0.8 (-1.1, 2.6) | -1.5 (-3.3, 0.3) | 0.7 (-1.2, 2.6) | -1.4 (-3.3, 0.4) |
| SHBG (nmol/L) | -1.9 (-3.6, -0.1) | -1.3 (-3.1, 0.5) | -1.8 (-3.5, -0.1) | -1.2 (-3.0, 0.6) |
| Animal protein d (%energy) | ||||
| Estradiol (pg/mL) | -2.0 (-6.8, 3.0) | 1.1 (-3.8, 6.3) | -0.7 (-5.6, 4.5) | 1.5 (-3.5, 6.8) |
| Progesterone (ng/mL)c | -6.5 (-20.9, 10.4) | 0.7 (-14.2, 18.2) | -1.6 (-16.8, 16.4) | -0.3 (-15, 16.8) |
| LH (ng/mL) | 0.8 (-4.5, 6.4) | 1.8 (-3.5, 7.3) | 1.1 (-4.4, 6.8) | 1.9 (-3.5, 7.5) |
| FSH (mIU/mL) | 1.2 (-2.4, 4.8) | 2.4 (-1.2, 6.1) | 1.3 (-2.3, 5.0) | 2.3 (-1.3, 6.1) |
| Testosterone (ng/dL) | 0.6 (-1.3, 2.5) | -1.0 (-2.8, 0.8) | 0.6 (-1.3, 2.5) | -0.7 (-2.5, 1.2) |
| SHBG (nmol/L) | -0.4 (-2.1, 1.4) | 0.2 (-1.5, 2.0) | -0.4 (-2.2, 1.4) | 0.2 (-1.6, 2.0) |
| Vegetable protein e (%energy) | ||||
| Estradiol (pg/mL) | 0.7 (-4.2, 5.8) | -4.4 (-9.2, 0.7) | -0.6 (-5.5, 4.6) | -4.0 (-9.0, 1.3) |
| Progesterone (ng/mL)c | -15.5 (-28.2, -0.6) | -0.7 (-15.6, 16.9) | -18.0 (-30.2, -3.6) | 13.6 (-3.9, 34.3) |
| LH (ng/mL) | 3.3 (-2.1, 8.9) | 0.8 (-4.6, 6.4) | 3.0 (-2.4, 8.8) | 0.5 (-5.0, 6.4) |
| FSH (mIU/mL) | 3.8 (0.2, 7.5) | 1.1 (-2.6, 4.9) | 3.8 (0.2, 7.6) | 1.4 (-2.4, 5.4) |
| Testosterone (ng/dL) | -0.3 (-2.1, 1.6) | 1.0 (-1.0, 2.9) | -0.1 (-2.0, 1.7) | 0.6 (-1.3, 2.6) |
| SHBG (nmol/L) | -0.1 (-1.8, 1.7) | 0.0 (-1.8, 1.9) | -0.1 (-1.9, 1.6) | 0.0 (-1.8, 1.9) |
All models were adjusted for prior hormone levels using inverse probability weights.
Abbreviations: BMI, body mass index; CI, confidence interval; FSH, follicle-stimulating hormone; LH, luteinizing hormone; SHBG, sex hormone-binding globulin.
a Energy from total protein intake, tertile 1: 3.5%–13.2% of energy; tertile 2: 13.2%–17.4% of energy; tertile 3: 17.4%–41.7% of energy. Energy from animal protein intake, tertile 1: 0.0%–7.5% of energy; tertile 2, 7.5%–12.1% of energy; tertile 3, 12.1%–40.7% of energy. Energy from vegetable protein intake, tertile 1, 0.4%–4.3% of energy; tertile 2, 4.3%–5.9% of energy; tertile 3, 5.9%–19.8% of energy.
b Adjusted for age, race, BMI, physical activity, alcohol, blood cadmium, mercury, and lead concentrations, and fiber and total energy intakes.
c Includes only measurements of progesterone during the luteal phase.
d Derived from consumption of red meats (beef, veal, lamb, pork, game, and organ meats), poultry, processed meats (cured pork, cold cuts, and sausage), fish (fish and shellfish), and eggs.
e Derived from consumption of meat alternatives (veggie burgers, tofu, tempeh, textured vegetable protein, and soy nut), legumes (dried beans, mature lima beans, refried beans, and beans in sauce and recipe), nuts and seeds, and nut and seed butters.
Table 3.
Weighted linear mixed models evaluating the association between substitution of daily protein intakes for carbohydrate, fat, and other types of protein, assessed up to 4 times per menstrual cycle (menstruation, midfollicular phase, expected ovulation, and midluteal phase) for 2 cycles,a and percent difference in log-transformed serum reproductive hormone concentrations measured up to 8 times per menstrual cycle for 2 cycles
| Carbohydrate Substitution Modelb | Fat Substitution Modelc | Protein Substitution Modeld | ||||
|---|---|---|---|---|---|---|
| Tertile 1 vs Tertile 2 | Tertile 3 vs Tertile 2 | Tertile 1 vs Tertile 2 | Tertile 3 vs Tertile 2 | Tertile 1 vs Tertile 2 | Tertile 3 vs Tertile 2 | |
| % Difference (95% CI) | % Difference (95% CI) | % Difference (95% CI) | % Difference (95% CI) | % Difference (95% CI) | % Difference (95% CI) | |
| Total protein (% energy) | ||||||
| Estradiol (pg/mL) | 0.3 (-4.6,5.5) | 1.7 (-3.4, 7.0) | 0.0 (-4.9, 5.2) | 2.1 (-3.0, 7.6) | – | – |
| Progesterone (ng/mL)e | -4.6 (-18.9, 12.3) | 1.0 (-13.6, 17.9) | -7.0 (-21.2, 9.6) | 2.8 (-12.2, 20.5) | – | – |
| LH (ng/mL) | -1.0 (-6.2, 4.5) | -2.2 (-7.3, 3.2) | -0.7 (-6.0, 4.8) | -2.6 (-7.7, 2.9) | – | – |
| FSH (mIU/mL) | 0.4 (-3.1, 4.1) | -0.5 (-4.0, 3.2) | 0.5 (-3.0, 4.1) | -0.5 (-4.1, 3.2) | – | – |
| Testosterone (ng/dL) | 0.7 (-1.1, 2.6) | -1.5 (-3.4, 0.3) | 0.9 (-1.0, 2.8) | -1.8 (-3.6, 0.1) | – | – |
| SHBG (nmol/L) | -1.8 (-3.5, -0.1) | -1.2 (-3.0, 0.6) | -1.8 (-3.5, -0.1) | -1.2 (-3.0, 0.6) | – | – |
| Animal protein f (%energy) | ||||||
| Estradiol (pg/mL) | -1.0 (-5.9, 4.2) | 1.7 (-3.3, 7.0) | -1.2 (-6.2, 4.0) | 2.1 (-3.0, 7.5) | -0.2 (-5.3,5.2) | 1.7 (-3.6,7.2) |
| Progesterone (ng/mL)f | -2.9 (-17.7, 14.7) | 1.3 (-13.5, 18.5) | -4.2 (-19.2, 13.4) | 3.1 (-12.3, 21.1) | -3.8 (-19,14.4) | 2.0 (-13.8,20.6) |
| LH (ng/mL) | 1.2 (-4.2, 7.0) | 1.8 (-3.6, 7.4) | 1.4 (-4.1, 7.2) | 1.5 (-3.9, 7.2) | 1.5 (-4.1,7.4) | 0.1 (-5.4,6.0) |
| FSH (mIU/mL) | 1.3 (-2.3, 5.0) | 2.3 (-1.3, 6.1) | 1.3 (-2.4, 5.0) | 2.3 (-1.3, 6.1) | 1.4 (-2.3,5.2) | 1.8 (-2.0,5.6) |
| Testosterone (ng/dL) | 0.7 (-1.2, 2.7) | -0.7 (-2.6, 1.1) | 0.8 (-1.1, 2.8) | -0.9 (-2.8, 0.9) | 0.7 (-1.2,2.7) | -0.9 (-2.8,1.0) |
| SHBG (nmol/L) | -0.4 (-2.2, 1.4) | 0.2 (-1.6, 2.0) | -0.4 (-2.2, 1.4) | 0.1 (-1.7, 1.9) | -0.5 (-2.3,1.3) | 0.4 (-1.4,2.3) |
| Vegetable protein g (%energy) | ||||||
| Estradiol (pg/mL) | -0.3 (-5.2, 4.9) | -4.2 (-9.2, 1.0) | -0.4 (-5.3, 4.8) | -4.2 (-9.2, 1.1) | -0.5 (-5.5,4.8) | -4.0 (-9.1,1.4) |
| Progesterone (ng/mL)f | -16.6 (-29.0, -2.0) | 11.4 (-5.6, 31.6) | -17.3 (-29.7, -2.8) | 12.3 (-5.1, 32.7) | -16.5 (-29.2,-1.6) | 13.1 (-4.5,33.8) |
| LH (ng/mL) | 2.9 (-2.5, 8.7) | 0.6 (-4.9, 6.5) | 2.9 (-2.5, 8.7) | 0.7 (-4.9, 6.6) | 2.6 (-2.9,8.5) | -0.1 (-5.7,5.9) |
| FSH (mIU/mL) | 3.8 (0.2, 7.6) | 1.4 (-2.4, 5.4) | 3.8 (0.2, 7.6) | 1.4 (-2.4, 5.4) | 3.9 (0.2,7.7) | 1.5 (-2.4,5.5) |
| Testosterone (ng/dL) | -0.2 (-2.1, 1.6) | 0.7 (-1.2, 2.7) | -0.3 (-2.1, 1.6) | 0.7 (-1.2, 2.7) | 0.1 (-1.7,2.0) | 0.6 (-1.3,2.6) |
| SHBG (nmol/L) | -0.2 (-1.9, 1.6) | 0.0 (-1.8, 1.9) | -0.2 (-1.9, 1.6) | 0.0 (-1.8, 1.9) | -0.3 (-2.1,1.5) | 0.2 (-1.6,2.1) |
All models were adjusted for prior hormone levels using inverse probability weights.
Abbreviations: BMI, body mass index; CI, confidence interval; FSH, follicle-stimulating hormone; LH, luteinizing hormone; SHBG, sex hormone-binding globulin.
a Energy from total protein intake, tertile 1: 3.5%–13.2% of energy; tertile 2: 13.2%–17.4% of energy; tertile 3: 17.4%–41.7% of energy. Energy from animal protein intake, tertile 1: 0.0%–7.5% of energy; tertile 2, 7.5%–12.1% of energy; tertile 3, 12.1%–40.7% of energy. Energy from vegetable protein intake, tertile 1, 0.4T–4.3% of energy; tertile 2, 4.3T–5.9% of energy; tertile 3, 5.9T–19.8% of energy.
b Adjusted for age, race, BMI, physical activity, alcohol, blood cadmium, mercury, and lead concentrations, and fiber, total energy, and fat intakes.
c Adjusted for age, race, BMI, physical activity, alcohol, blood cadmium, mercury, and lead concentrations, and fiber, total energy, and carbohydrate intakes.
d Adjusted for age, race, BMI, physical activity, alcohol, blood cadmium, mercury, and lead concentrations, and fiber, total energy, fat, carbohydrate, and other types of protein intakes (ie, animal protein models were adjusted for vegetable protein and vegetable protein models were adjusted for animal protein).
e Includes only measurements of progesterone during the luteal phase.
f Derived from consumption of red meats (beef, veal, lamb, pork, game, and organ meats), poultry, processed meats (cured pork, cold cuts, and sausage), fish (fish and shellfish), and eggs.
g Derived from consumption of meat alternatives (veggie burgers, tofu, tempeh, textured vegetable protein, and soy nut), legumes (dried beans, mature lima beans, refried beans, and beans in sauce and recipe), nuts and seeds, and nut and seed butters.
Our data did not suggest associations between total or animal protein intakes and anovulation after adjusting for potential confounders (Table 4). However, the lowest tertile of vegetable protein intake, compared with the middle tertile, was associated with an increased risk of anovulation (RR 2.53, 95% CI 1.21, 5.26), whereas no associations were indicated for higher intakes of vegetable protein. Results were similar for all substitution models.
Table 4.
Generalized linear models evaluating the association between the average protein intakes before ovulation (menstruation, midfollicular phase)a and risk of anovulation
| Unadjusted | Adjustedb | Carbohydrate Substitution Modelc | Fat Substitution Modeld | Protein Substitution Modele | |||
|---|---|---|---|---|---|---|---|
| Anovulatory n (%) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |||
| Total protein (% energy) | Tertile 1 | 16 (38) | 0.93 (0.52, 1.68) | 0.83 (0.45, 1.51) | 0.73 (0.38, 1.42) | 0.77 (0.38, 1.56) | – |
| Tertile 2 | 18 (43) | Reference | Reference | Reference | Reference | – | |
| Tertile 3 | 8 (19) | 0.45 (0.23, 0.88) | 0.65 (0.34, 1.24) | 0.56 (0.28, 1.14) | 0.52 (0.25, 1.08) | – | |
| Animal protein f (% energy) | Tertile 1 | 16 (38) | 1.00 (0.56, 1.79) | 0.98 (0.46, 2.06) | 0.98 (0.46, 2.08) | 1.01 (0.47, 2.19) | 1.11 (0.49, 2.48) |
| Tertile 2 | 16 (38) | Reference | Reference | Reference | Reference | Reference | |
| Tertile 3 | 10 (24) | 0.59 (0.30, 1.13) | 0.81 (0.39, 1.68) | 0.81 (0.39, 1.68) | 0.78 (0.36, 1.67) | 0.67 (0.24, 1.89) | |
| Vegetable protein g (% energy) | Tertile 1 | 17 (40) | 2.05 (1.08, 3.90) | 2.53 (1.21, 5.26) | 2.51 (1.21, 5.20) | 2.52 (1.21, 5.26) | 2.65 (1.24, 5.63) |
| Tertile 2 | 9 (21) | Reference | Reference | Reference | Reference | Reference | |
| Tertile 3 | 16 (38) | 1.52 (0.82, 2.82) | 0.95 (0.44, 2.05) | 0.95 (0.43, 2.10) | 0.95 (0.43, 2.08) | 0.90 (0.40, 2.00) |
Abbreviations: BMI, body mass index; CI, confidence interval; RR, risk ratio.
a Mean energy from total protein intake before ovulation, tertile 1: 4.3%–13.3% of energy; tertile 2: 13.3%–16.4% of energy; tertile 3: 16.4%–36.3% of energy. Mean energy from animal protein intake, tertile 1: 0.1%–7.9% of energy; tertile 2, 7.9%–11.2% of energy; tertile 3, 11.2%–34.4% of energy. Mean energy from vegetable protein intake, tertile 1, 1.6%–4.5% of energy; tertile 2, 4.5%–5.9% of energy; tertile 3, 5.9%–19.2% of energy.
b Adjusted for age, race, BMI, physical activity, alcohol, blood cadmium, mercury, and lead concentrations, and fiber and total energy intakes.
c Adjusted for age, race, BMI, physical activity, alcohol, blood cadmium, mercury, and lead concentrations, and fiber, total energy, and fat intakes.
d Adjusted for age, race, BMI, physical activity, alcohol, blood cadmium, mercury, and lead concentrations, and fiber, total energy, and carbohydrate intakes.
e Adjusted for age, race, BMI, physical activity, alcohol, blood cadmium, mercury, and lead concentrations, and fiber, total energy, fat, carbohydrate, and other types of protein intakes (ie, animal protein models were adjusted for vegetable protein and vegetable protein models were adjusted for animal protein).
f Derived from consumption of red meats (beef, veal, lamb, pork, game, and organ meats), poultry, processed meats (cured pork, cold cuts, and sausage), fish (fish and shellfish), and eggs.
g Derived from consumption of meat alternatives (veggie burgers, tofu, tempeh, textured vegetable protein, and soy nut), legumes (dried beans, mature lima beans, refried beans, and beans in sauce and recipe), nuts and seeds, and nut and seed butters.
In general, intake of meat, including red meat, poultry, and processed meat, was not associated with reproductive hormones (Table 5). However, associations between other animal-based protein-rich foods and certain hormones were observed. In particular, egg intake, compared to no intake, was associated with higher FSH concentrations (4.6% difference, 95% CI 0.2, 9.1). Among protein-rich foods from vegetable sources, intake of legumes was associated with higher progesterone concentrations (22.3% difference, 95% CI 5.2, 42.1) and intake of nuts and seeds was suggestive of lower FSH concentrations (-4.0% difference, 95% CI -8.7, 1.0, compared with no intake). Intake of legumes was suggestive of a 47% lower risk of anovulation (RR 0.53, 95% CI 0.22, 1.29), whereas intake of nuts and seeds was associated with an elevated risk of anovulation (RR 2.12, 95% CI 1.17, 3.85, compared with no intake) (Table 6).
Table 5.
Weighted linear mixed models evaluating the association between the average intakes of protein-rich foods across menstrual cycle (menstruation, midfollicular phase, expected ovulation, and midluteal phase) and log-transformed serum reproductive hormone concentrations measured up to 8 times per menstrual cycle for 2 cycles
| Unadjusted | Adjusteda | |
|---|---|---|
| Protein food groups (per serving) | % Difference (95% CI) | % Difference (95% CI) |
| All meats (median serving 2.98/day) | ||
| Estradiol (pg/mL) | 0.5 (-0.8, 1.9) | -0.1 (-1.6, 1.5) |
| Progesterone (ng/mL)b | 0.4 (-3.1, 4.1) | -1.2 (-5.2, 3.0) |
| LH (ng/mL) | -0.2 (-1.7, 1.2) | -0.1 (-1.7, 1.6) |
| FSH (mIU/mL) | 0.7 (-0.5, 1.8) | 0.6 (-0.7, 1.9) |
| Testosterone (ng/dL) | -0.2 (-0.8, 0.5) | 0.0 (-0.7, 0.8) |
| SHBG (nmol/L) | -0.3 (-0.8, 0.2) | 0.0 (-0.6, 0.6) |
| Red meats (median serving 0.52/day) | ||
| Estradiol (pg/mL) | 1.0 (-1.3, 3.4) | 0.3 (-2.1, 2.7) |
| Progesterone (ng/mL)b | -2.0 (-8.0, 4.4) | -4.8 (-10.9, 1.7) |
| LH (ng/mL) | 0.4 (-2.0, 2.9) | 0.4 (-2.1, 3.1) |
| FSH (mIU/mL) | 0.4 (-1.6, 2.3) | -0.2 (-2.2, 1.8) |
| Testosterone (ng/dL) | -0.5 (-1.7, 0.6) | -0.4 (-1.5, 0.8) |
| SHBG (nmol/L) | -0.1 (-1.6, 1.4) | 0.0 (-1.6, 1.5) |
| Poultry (median serving 1.27/day) | ||
| Estradiol (pg/mL) | 0.3 (-1.4, 2.1) | 0.0 (-1.9, 1.9) |
| Progesterone (ng/mL)b | 1.8 (-2.9, 6.8) | 1.2 (-3.7, 6.4) |
| LH (ng/mL) | -0.3 (-2.1, 1.6) | 0.0 (-2.0, 2.0) |
| FSH (mIU/mL) | 0.6 (-0.8, 2.1) | 0.9 (-0.7, 2.4) |
| Testosterone (ng/dL) | 0.4 (-0.4, 1.3) | 0.6 (-0.3, 1.5) |
| SHBG (nmol/L) | 0.3 (-0.8, 1.4) | 0.7 (-0.5, 1.9) |
| Processed meats (median serving 0.46/day) | ||
| Estradiol (pg/mL) | -0.2 (-3.7, 3.5) | -0.6 (-4.2, 3.2) |
| Progesterone (ng/mL)b | 1.4 (-7.8, 11.6) | 0.9 (-8.6, 11.4) |
| LH (ng/mL) | -1.7 (-5.3, 2.2) | -1.3 (-5.1, 2.8) |
| FSH (mIU/mL) | 0.6 (-2.4, 3.7) | -0.1 (-3.1, 3.1) |
| Testosterone (ng/dL) | -1.2 (-3.1, 0.6) | -0.9 (-2.7, 1.0) |
| SHBG (nmol/L) | 0.7 (-0.7, 2.2) | 0.5 (-1.9, 3.1) |
| Fish c (serving 41%) | ||
| Estradiol (pg/mL) | 4.1 (-1.2, 9.7) | 2.4 (-2.9, 8.1) |
| Progesterone (ng/mL)b | 14.1 (-1, 31.6) | 9.9 (-5.0, 27.0) |
| LH (ng/mL) | -0.5 (-5.9, 5.1) | -0.3 (-5.8, 5.6) |
| FSH (mIU/mL) | -0.8 (-5.1, 3.7) | -1.2 (-5.5, 3.4) |
| Testosterone (ng/dL) | 0.1 (-2.6, 2.7) | -0.1 (-2.7, 2.6) |
| SHBG (nmol/L) | -1.8 (-3.7, 0.2) | -1.9 (-5.2, 1.5) |
| Eggs (serving 64%) | ||
| Estradiol (pg/mL) | 0.2 (-4.7, 5.3) | -1.0 (-6.0, 4.3) |
| Progesterone (ng/mL)b | -7.1 (-19.4,7 .0) | -10.9 (-22.8, 2.9) |
| LH (ng/mL) | 3.8 (-1.6, 9.6) | 3.1 (-2.5, 9.0) |
| FSH (mIU/mL) | 4.2 (-0.1, 8.6) | 4.6 (0.2, 9.1) |
| Testosterone (ng/dL) | 1.3 (-1.1, 3.8) | 1.9 (-0.6, 4.4) |
| SHBG (nmol/L) | -1.1 (-2.9, 0.7) | 0.1 (-3.2, 3.5) |
| Meat alternatives c (serving 17%) | ||
| Estradiol (pg/mL) | -1.4 (-8.0, 5.7) | -1.7 (-8.4, 5.4) |
| Progesterone (ng/mL)b | 3.6 (-14.1, 24.8) | 3.1 (-14.3, 24.1) |
| LH (ng/mL) | 1.8 (-5.5, 9.6) | 1.9 (-5.5, 9.8) |
| FSH (mIU/mL) | -1.6 (-7.1, 4.2) | -3.6 (-9.0, 2.1) |
| Testosterone (ng/dL) | 3.2 (-0.3, 6.7) | 2.9 (-0.5, 6.5) |
| SHBG (nmol/L) | -0.9 (-5.3, 3.6) | -1.1 (-5.5, 3.4) |
| Legumes c (serving 37%) | ||
| Estradiol (pg/mL) | 1.3 (-4.0, 6.8) | 2.7 (-2.9, 8.6) |
| Progesterone (ng/mL)b | 17.1 (1.4, 35.2) | 22.3 (5.2, 42.1) |
| LH (ng/mL) | -0.8 (-6.2, 5.0) | -0.5 (-6.3, 5.6) |
| FSH (mIU/mL) | 2.6 (-1.9, 7.3) | 3.0 (-1.7, 7.9) |
| Testosterone (ng/dL) | -2.0 (-4.5, 0.7) | -2.3 (-4.9, 0.3) |
| SHBG (nmol/L) | -0.1 (-3.6, 3.4) | 0.0 (-3.5, 3.7) |
| Nuts and seeds c (serving 27%) | ||
| Estradiol (pg/mL) | -1.4 (-7.0, 4.4) | -0.9 (-6.7, 5.2) |
| Progesterone (ng/mL)b | 1.6 (-13.1, 18.8) | 0.7 (-14.3, 18.5) |
| LH (ng/mL) | -1.6 (-7.5, 4.6) | -2.1 (-8.2, 4.5) |
| FSH (mIU/mL) | -2.5 (-7.2, 2.3) | -4.0 (-8.7, 1.0) |
| Testosterone (ng/dL) | 0.7 (-2.1, 3.6) | 0.5 (-2.4, 3.5) |
| SHBG (nmol/L) | 2.1 (-1.7, 6.0) | 1.9 (-2.0, 5.9) |
| Nut and seed butters c (serving 23%) | ||
| Estradiol (pg/mL) | -0.8 (-7.0, 5.9) | 2.4 (-4.3, 9.6) |
| Progesterone (ng/mL)b | 3.5 (-12.9, 23.1) | 8.8 (-9.2, 30.5) |
| LH (ng/mL) | -3.4 (-9.7, 3.5) | -6.1 (-12.6, 0.9) |
| FSH (mIU/mL) | -3.1 (-8.3, 2.3) | -2.7 (-8.1, 2.9) |
| Testosterone (ng/dL) | -1.7 (-4.9, 1.6) | -2.9 (-6.1, 0.5) |
| SHBG (nmol/L) | -1.1 (-5.3, 3.4) | -1.3 (-5.7, 3.2) |
All models were adjusted for prior hormone levels using inverse probability weights.
Abbreviations: BMI, body mass index; CI, confidence interval; FSH, follicle-stimulating hormone; LH, luteinizing hormone; SHBG, sex hormone-binding globulin.
a Adjusted for age, race, BMI, physical activity, alcohol, blood cadmium, mercury, and lead concentrations, and intakes of total energy, fiber, and fat.
b Includes only measurements of progesterone during the luteal phase.
c Calculated for any versus no intakes (reference).
Table 6.
Generalized linear models evaluating the association between the average intakes of protein-rich foods before ovulation and risk of anovulation
| Unadjusted | Adjusteda | |
|---|---|---|
| Protein food groups (servings) | RR (95% CI) | RR (95% CI) |
| All meatsb (median serving 2.8/day) | 0.96 (0.82, 1.12) | 0.97 (0.81, 1.17) |
| Red meatsb (median serving 0.0/day) | 0.94 (0.74, 1.20) | 0.96 (0.74, 1.25) |
| Poultryb (median serving 1.0/day) | 1.01 (0.83, 1.21) | 1.02 (0.82, 1.27) |
| Processed meatsb (median serving 0.2/day) | 0.81 (0.53, 1.22) | 0.78 (0.48, 1.26) |
| Fishc (22% >0 serving/day) | 0.99 (0.56, 1.74) | 1.12 (0.58, 2.14) |
| Eggsc (44% >0 serving/day) | 1.15 (0.67, 1.96) | 1.20 (0.68, 2.12) |
| Meat alternativesc (9% >0 serving/day) | 1.27 (0.52, 3.09) | 1.26 (0.50, 3.17) |
| Legumesc (23% >0 serving/day) | 0.83 (0.44, 1.58) | 0.53 (0.22, 1.29) |
| Nuts and seedsc (17% >0 serving/day) | 1.67 (0.92, 3.01) | 2.12 (1.17, 3.85) |
| Nut and seed buttersc (14% >0 serving/day) | 0.86 (0.34, 2.17) | 0.92 (0.38, 2.22) |
Abbreviations: BMI, body mass index; CI, confidence interval; RR, risk ratio.
a Adjusted for age, race, BMI, physical activity, alcohol, blood cadmium, mercury, and lead concentrations, and intakes of total energy, fiber, and fat.
b Assessed per serving increase.
c Calculated for any versus no intakes (reference).
Discussion
Among healthy premenopausal women, lower intakes of vegetable protein were associated with changes in reproductive hormone concentrations and an elevated risk of sporadic anovulation. However, total and animal protein intakes were not associated with ovulatory function, even after accounting for important confounding factors including markers of environmental contaminants. Given increasing trends toward plant-based diets and growing interests regarding their impact on reproductive health, these findings shed light on the importance of adequate vegetable protein intake for ovulatory function among a sample of women largely meeting the recommended dietary allowance for total protein and following nonvegetarian diets.
Studies to date regarding dietary protein intake and its potential role on changes in reproductive hormones have primarily focused on comparisons between vegetarian and nonvegetarian diets, with conflicting results (9, 10). Specifically, one study observed a higher proportion of anovulatory cycles and lower serum concentrations of LH, estradiol, and progesterone in women following vegetarian diets compared to nonvegetarian diets (9), whereas another study found fewer anovulatory cycles among vegetarians (10). A direct comparison with our result is not feasible, as no participants in our cohort were following vegetarian diets by design, and the amount of vegetable protein intake was not quantified in those prior studies. Yet, our findings suggesting that low vegetable protein intake may inhibit ovulation are in line with the latter study (10), as both suggest a potential role of vegetable protein for normal ovulatory function. Our results are also in partial agreement with the findings from the Nurses’ Health Study II cohort, whereby protective associations of ovulatory infertility with vegetable protein, but not specific food sources of vegetable protein, were observed (8). Specifically, our data suggested elevated risk of anovulation with intakes of nuts and seeds, whereas no such associations with any particular foods rich in vegetable protein were found in that study (8). Of note, our findings are unexpected, as these are sources of omega-3 fatty acids and antioxidants. However, these findings may be related to a small increase in anovulation risk with higher concentrations of serum γ-tocopherol, a lipid soluble micronutrient mainly obtained from nuts and seeds, in this cohort of women (22). Nevertheless, cautious interpretation is warranted.
A few prior studies have suggested potential links between animal protein and reproductive outcomes. In the aforementioned Nurses’ Health Study II cohort, higher consumption of animal protein was associated with an elevated risk of ovulatory infertility (8). On the other hand, among women undergoing infertility treatment with assisted reproductive technologies, higher fish consumption was associated with a higher probability of live birth (23). Such links observed in prior studies were not evident in our study, as consumption of animal protein was largely not associated with differences in reproductive hormones and anovulation. However, it is possible that our null and weak findings could be related in part to relatively lower overall intake of animal protein of women in this study compared to other studies (8, 23, 24). Thus, further investigation to confirm these findings among women who consume animal protein, particularly red meat, poultry, and fish, in larger quantities is warranted.
Specific mechanisms that may explain associations between dietary protein and reproductive hormones are not clear, though they could be related to the role of proteins in cellular metabolism, tissue maintenance, and hormone synthesis. A mechanism through interaction with SHBG might also be related to our findings, as bioavailability of sex hormones, including estradiol and testosterone, is influenced by SHBG. This was indeed suggested in a study among postmenopausal women where consumption of red meat was associated with decreased SHBG and subsequently increased estradiol concentrations (25). However, though a small decrease in SHBG was observed for lower intake of total protein in our data, this did not translate into substantial differences in other hormones.
This study has several strengths and limitations. Multiple longitudinal measures of dietary protein intake along with reproductive hormone measurements across the menstrual cycle are unique strengths of our study. We have previously reported that the consumption of protein varied across the cycle in BioCycle (19). As such, our prospective measurement of diet using the multiple validated 24-hour dietary recalls at specific phases of the menstrual cycle may reduce the potential for misclassification (26, 27) and provides an ideal setting to investigate associations with reproductive hormones which also change over the cycle. However, as dietary intake was assessed for only 2 menstrual cycles, our findings reflect short-term associations between protein intake and ovulatory function. At enrollment, study participants were not planning to undertake special diets, including vegetarian diets. This is likely reflected in the relatively low intake of soy-rich foods in our cohort, which is typically considered as a major source of vegetable protein among vegetarians. In our prior work in this cohort, we did not find that isoflavones were associated with reproductive hormones or anovulation (28), which may be due to the low levels of intake. Based on this prior work, it is unlikely that isoflavones would be driving the observed findings with total vegetable protein intake. During the study period, approximately 84% of the women consumed total protein above the RDA set for reproductive aged women, which enhances the generalizability of our findings for healthy women following regular diets. As daily measures of progesterone or transvaginal ultrasounds were unavailable to confirm ovulation, misclassification of anovulation is possible. However, multiple well-timed serum hormone measurements (ie, up to 8 times per cycle) were used to classify ovulatory cycles. Importantly, these analyses were adjusted for blood measurements of heavy metals (ie, cadmium, lead, and mercury) to account for the potential influence from consuming contaminated foods, which could impact reproductive hormone concentrations.
Conclusions
Given the trend toward reducing consumption of animal products and increasing plant-based diets, understanding the role of dietary protein from different food sources for ovulatory function is important. It is particularly critical for reproductive-aged women as it may help identify modifiable dietary contributors for women’s reproductive health and fertility. We addressed this important question using longitudinally collected dietary protein for both types and food sources and hormone data that were timed at specific phases across a menstrual cycle. Further, we adjusted for environmental contaminants, accounting for their possible impact on associations between intakes of dietary protein through various food sources and ovulatory function. Overall, our results suggest that low intake of vegetable protein, irrespective of intake of other macronutrients or animal proteins, may be associated with differences in reproductive hormone concentrations across the menstrual cycle and risk of sporadic anovulation in healthy women meeting the recommended daily protein requirements and not following restricted diets.
Acknowledgments
Financial Support. This research was supported by the Intramural Research Program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development (Contract Numbers: HHSN275200403394C, HHSN275201100002I, and Task 1 HHSN27500001).
Author Contributions: KK and SLM designed and conducted the research; KK analyzed the data; KK, SFY, CJN, VCA, and EAD wrote the paper; LAS, AA, NJP, and SLM critically revised the paper; KK and SLM had the primary responsibility for the final content. All authors read and approved the final manuscript.
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Data on demographics and reproductive hormones analyzed during this study are available at the Data and Specimen Hub (DASH) of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (https://dash.nichd.nih.gov/). Data on dietary intake analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Key TJ, Appleby PN, Bradbury KE, et al. . Consumption of meat, fish, dairy products, and eggs and risk of ischemic heart disease. Circulation. 2019;139(25):2835-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pan A, Sun Q, Bernstein AM, et al. . Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr. 2011;94(4):1088-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Willett WC, Stampfer MJ, Colditz GA, Rosner BA, Speizer FE. Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N Engl J Med. 1990;323(24):1664-1672. [DOI] [PubMed] [Google Scholar]
- 4. Fraser AJ, Webster TF, McClean MD. Diet contributes significantly to the body burden of PBDEs in the general U.S. population. Environ Health Perspect. 2009;117(10):1520-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jeong SH, Kang D, Lim MW, Kang CS, Sung HJ. Risk assessment of growth hormones and antimicrobial residues in meat. Toxicol Res. 2010;26(4):301-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Willett W, Rockström J, Loken B, et al. . Food in the Anthropocene: the EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet. 2019;393(10170):447-492. [DOI] [PubMed] [Google Scholar]
- 7. Yamamoto A, Harris HR, Vitonis AF, Chavarro JE, Missmer SA. A prospective cohort study of meat and fish consumption and endometriosis risk. Am J Obstet Gynecol. 2018;219(2):178.e1-178.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Protein intake and ovulatory infertility. Am J Obstet Gynecol. 2008;198(2):210.e1-210.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pirke KM, Schweiger U, Laessle R, Dickhaut B, Schweiger M, Waechtler M. Dieting influences the menstrual cycle: vegetarian versus nonvegetarian diet. Fertil Steril. 1986;46(6):1083-1088. [PubMed] [Google Scholar]
- 10. Barr SI, Janelle KC, Prior JC. Vegetarian vs nonvegetarian diets, dietary restraint, and subclinical ovulatory disturbances: prospective 6-mo study. Am J Clin Nutr. 1994;60(6):887-894. [DOI] [PubMed] [Google Scholar]
- 11. Kim K, Wactawski-Wende J, Michels KA, et al. . Dairy food intake is associated with reproductive hormones and sporadic anovulation among healthy premenopausal women. J Nutr. 2017;147(2):218-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pasiakos SM, Agarwal S, Lieberman HR, Fulgoni VL 3rd. Sources and Amounts of Animal, Dairy, and Plant Protein Intake of US Adults in 2007-2010. Nutrients. 2015;7(8):7058-7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wactawski-Wende J, Schisterman EF, Hovey KM, et al. ; BioCycle Study Group . BioCycle study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatr Perinat Epidemiol. 2009;23(2):171-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howards PP, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, Hovey KM. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. Am J Epidemiol. 2009;169(1):105-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lynch KE, Mumford SL, Schliep KC, et al. . Assessment of anovulation in eumenorrheic women: comparison of ovulation detection algorithms. Fertil Steril. 2014;102(2):511-518.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Craig CL, Marshall AL, Sjöström M, et al. . International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-1395. [DOI] [PubMed] [Google Scholar]
- 17. Institutue of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Choleterol, Protein, and Amino Acids. Washington DC: National Academies Press; 2002. [DOI] [PubMed] [Google Scholar]
- 18. Fulgoni VL, 3rd. Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003–2004. Am J Clin Nutr 2008;87(5):1554s-1557s. [DOI] [PubMed] [Google Scholar]
- 19. Gorczyca AM, Sjaarda LA, Mitchell EM, et al. . Changes in macronutrient, micronutrient, and food group intakes throughout the menstrual cycle in healthy, premenopausal women. Eur J Nutr. 2016;55(3):1181-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Willett W. Nutritional Epidemiology. New York: Oxford University Press; 2013. [Google Scholar]
- 22. Mumford SL, Browne RW, Schliep KC, et al. . Serum antioxidants are associated with serum reproductive hormones and ovulation among healthy women. J Nutr. 2016;146(1):98-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nassan FL, Chiu YH, Vanegas JC, et al. ; EARTH Study Team . Intake of protein-rich foods in relation to outcomes of infertility treatment with assisted reproductive technologies. Am J Clin Nutr. 2018;108(5):1104-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Souter I, Chiu YH, Batsis M, et al. ; EARTH Study Team . The association of protein intake (amount and type) with ovarian antral follicle counts among infertile women: results from the EARTH prospective study cohort. BJOG. 2017;124(10):1547-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brinkman MT, Baglietto L, Krishnan K, et al. . Consumption of animal products, their nutrient components and postmenopausal circulating steroid hormone concentrations. Eur J Clin Nutr. 2010;64(2):176-183. [DOI] [PubMed] [Google Scholar]
- 26. Schatzkin A, Kipnis V, Carroll RJ, et al. . A comparison of a food frequency questionnaire with a 24-hour recall for use in an epidemiological cohort study: results from the biomarker-based Observing Protein and Energy Nutrition (OPEN) study. Int J Epidemiol. 2003;32(6):1054-1062. [DOI] [PubMed] [Google Scholar]
- 27. Subar AF, Kipnis V, Troiano RP, et al. . Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol. 2003;158(1):1-13. [DOI] [PubMed] [Google Scholar]
- 28. Filiberto AC, Mumford SL, Pollack AZ, et al. . Usual dietary isoflavone intake and reproductive function across the menstrual cycle. Fertil Steril. 2013;100(6):1727-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data on demographics and reproductive hormones analyzed during this study are available at the Data and Specimen Hub (DASH) of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (https://dash.nichd.nih.gov/). Data on dietary intake analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.