Fig. 5. In vivo targeting, safety, and therapeutic efficacy.
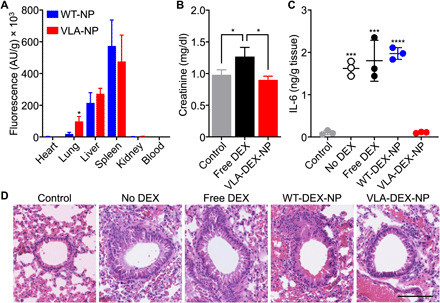
(A) Biodistribution of WT-NP or VLA-NP in a lung inflammation model 6 hours after intravenous administration (n = 3, mean + SD). *P < 0.05, Student’s t test. AU, arbitrary units. (B) Creatinine levels in the plasma of mice after repeated daily administrations for 9 days with free DEX or VLA-DEX-NP (n = 3, mean + SD). *P < 0.05, one-way ANOVA. (C) IL-6 levels in the lung tissue of mice intratracheally challenged with LPS and then treated intravenously with vehicle solution, free DEX, WT-DEX-NP, or VLA-DEX-NP (n = 3, mean ± SD). ***P < 0.001, ****P < 0.0001 (compared to VLA-DEX-NP), one-way ANOVA. (D) Representative hematoxylin and eosin–stained lung histology sections of mice intratracheally challenged with LPS and then treated intravenously with vehicle solution, free DEX, WT-DEX-NP, or VLA-DEX-NP (scale bar, 100 μm).
