FIGURE 1.
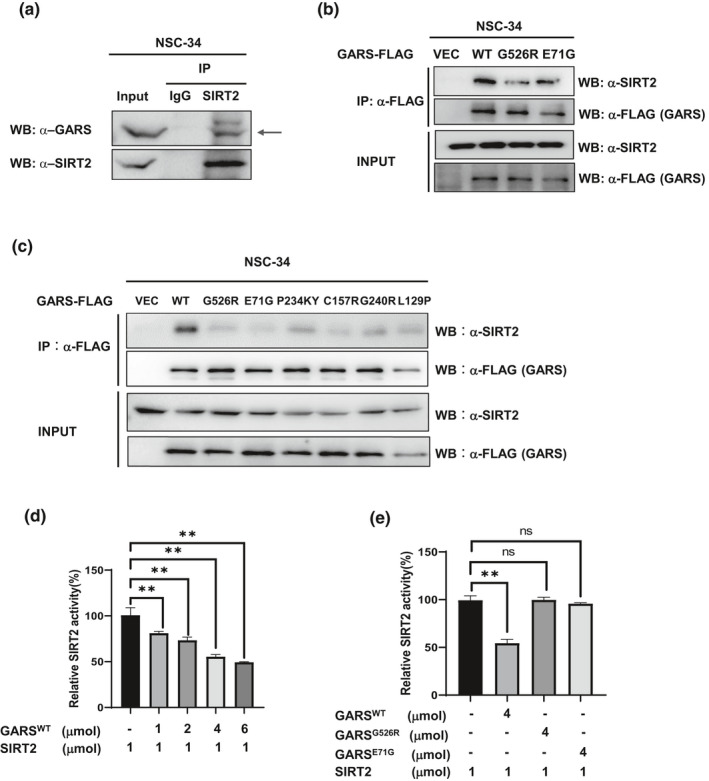
Wild‐type GlyRS tightly binds the SIRT2 to inhibit its activity, not GlyRSCMT2D. (a) Coimmunoprecipitation of endogenous sirt2 showing specifically interaction with endogenous GlyRS in NSC‐34 cells. (b) Representative immunoblotting of 3 independent experiments shows that SIRT2 interacts with wild‐type GlyRS, not GlyRS mutant in stably expressing FLAG‐tagged GlyRS NSC34 cells FLAG‐tagged GlyRS was stably expressed in NSC34 cells. Precipitated GlyRS‐FLAG (WT, G526R, E71G) was detected by anti‐FLAG antibody, and co‐IP endogenous SIRT2 was detected by anti‐SIRT2 as indicated. (c) Representative immunoblotting of 3 independent experiments shows that SIRT2 interacts with wild‐type GlyRS, not GlyRS mutants in NSC‐34 cells. The NSC‐34 cells were transfected with GlyRS‐FLAG (WT, G526R, E71G, P234KY, C157R, G240R, L129P). Coimmunoprecipitations were performed with anti‐FLAG M2 magnetic beads. The immunoblot analysis was performed with anti‐Sirt2 and anti‐FLAG. (d‐e) Effect of GlyRS(d) or GlyRS mutant (G526R, E71G) (e) on SIRT2 deacetylation activity. SIRT2 (1 µM) was incubated with purified GlyRS or GlyRS (G526R, E71G) (concentration measured as a monomer) at the indicated ratios. The deacetylase activities of recombinant human SIRT2 were measured by monitoring the fluorescence intensity (excitation at 360 nm and emission at 460 nm) using a substrate peptide with one end coupled to a fluorophore and the other end to a quencher. A reaction without NAD+ was performed as a negative control. Data are presented as mean ± SD, n = 3 biological replicates per group, from three independent experiments
