FIGURE 2.
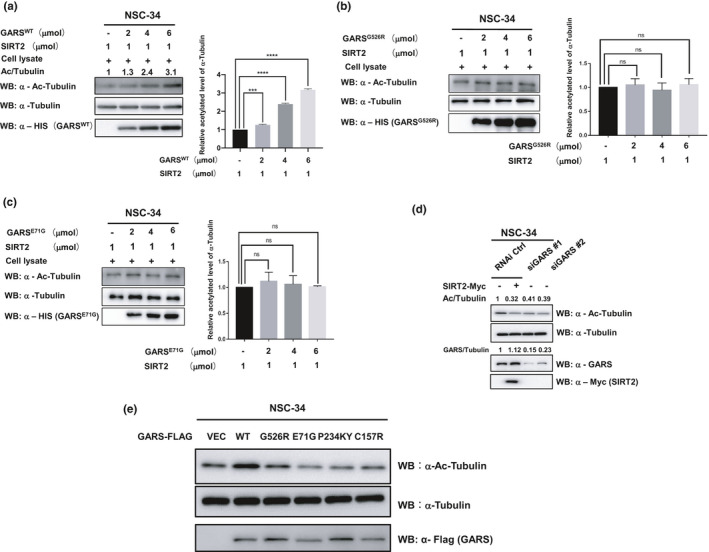
Wild‐type GlyRS binds the SIRT2 to maintain the acetylated a‐tubulin. (a–c) The recombinant SIRT2(1µmol) and recombinant GlyRSWT (a), GlyRSG526R (b), or GlyRSE71G (c) were incubated with NSC‐34 lysate at the indicated ratios(concentration measured as a monomer). The reaction products were detected by Western blotting for acetylated tubulin, a‐tubulin, and His‐tag. Data are presented as mean ± SD, n = 3 biological replicates per group, from three independent experiments. (d) Representative immunoblotting of 3 independent experiments shows that both knockdowns of GlyRS and overexpression of SIRT2 result in decreased tubulin acetylation. HEK293 cells were transfected with GlyRS siRNA or control siRNA 24 h later, and SIRT2‐Myc was overexpressed in cells transfected with control siRNA. After an additional 48 h, cells were harvested and acetylation of tubulin was detected by Western blot. (e) Western blot analysis detecting the level of α‐tubulin acetylation in NSC‐34 cells transfected with wild‐type GlyRS and GlyRS mutants
