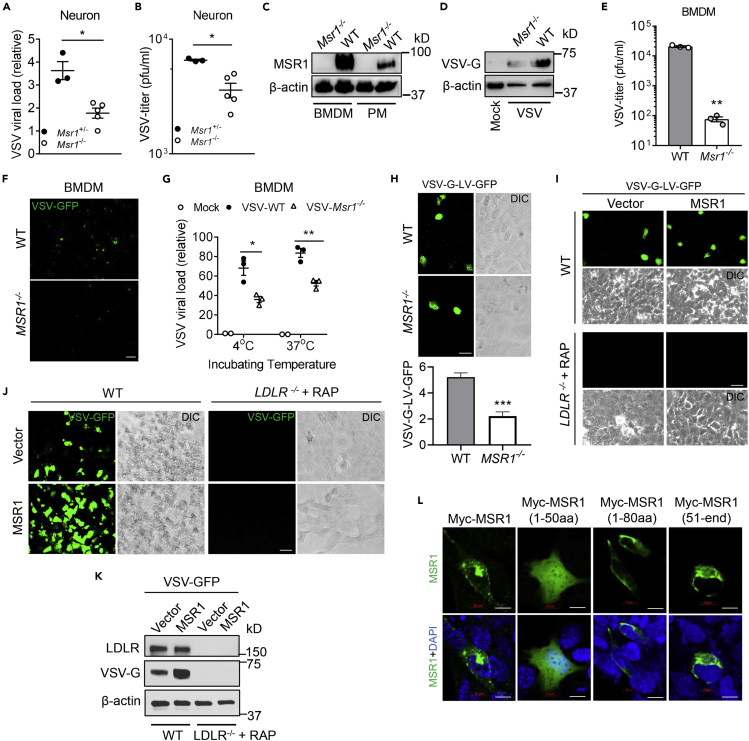
Figure 4.
MSR1 mediates cellular entry of VSV in primary mouse cells and human cells
(A and B) VSV loads in primary neurons of Msr1+/− and Msr1−/− littermates at 36 h after inoculation (MOI = 1, multiplicity of infection = 3), as assessed by (A) quantitative RT-PCR (expressed as a fold change over the lowest viral load) and (B) plaque forming assay. Each dot represents an individual mouse.
(C) Immunoblots of MSR1 protein expression in WT and Msr1−/− bone-marrow-derived macrophages (BMDMs) and peritoneal macrophages (PMs).
(D–F) (D) VSV-G protein expression in BMDMs, (E) virus titer in culture medium, and (F) VSV-GFP fluorescence under microscopy in BMDMs at 48 h after VSV-GFP infection (MOI = 5), N = 3 biological replicates. Objective: 20×, scale bar: 100 μm.
(G) Quantitative RT-PCR analyses of VSV virions attached to BMDMs (4°C for 2 h) and entry into BMDMs (37°C for 30 min). The cells were washed 3 times with cold PBS before switching from 4°C to 37°C and after 37°C as well. MOI = 10, N = 3 biological replicates.
(H) GFP expression in WT and MSR1−/− trophoblasts, 24 h after transduction with GFP-encoding VSV-G-pseudotyped lentiviral vectors (VSV-G-LV-GFP) without polybrene. Scale bar: 50 μm. The statistic VSV-G-LV-GFP was acquired with a fluorescence microscope from 9 random fields of three biological replicates.
(I) GFP expression in WT and LDLR−/−+ RAP trophoblasts, 24 h after transduction with VSV-G-LV-GFP. The LDLR−/− trophoblasts were pre-treated with RAP (200 nM, 30 min, 37°C) followed by VSV-G-LV-GFP transduction.
(J and K) The GFP fluorescence (J) and immunoblots of protein level (K) in VSV-GFP-infected WT and LDLR−/−+RAP trophoblasts with or without MSR1 overexpression; the WT and LDLR−/− trophoblasts were transfected with human MSR1 plasmid for 24 h, followed by VSV-GFP infection for 18 h (MOI = 0.5). The LDLR−/− trophoblasts were pre-treated with RAP (200 nM, 30 min, 37°C) before inoculation of VSV-GFP. Scale bar: 50 μm.
(L) Immunofluorescence staining for different Myc-tagged MSR1 fragments in human trophoblasts, 24 h after transfection of plasmids. Myc proteins were stained by a mouse anti-Myc antibody, followed by an Alexa Fluor 488 (green)-conjugated secondary antibody. The cell nuclei were stained by DAPI (blue). Scale bar: 10 μm. β-actin is a housekeeping control. Mock: no virus infection control. All the data are presented as mean ± S.E.M., and statistical significances are analyzed by a standard two-tailed unpaired Student's t-test, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.