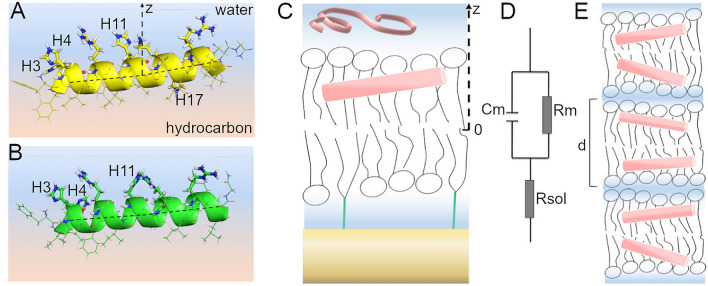
Figure 2.
Techniques for investigating peptide-bilayer structural interactions. (A) High-resolution structure of piscidin P1: FFHHIFRGIVHVGKTIHRLVTG (MW 2571) and (B) Piscidin P3: FIHHIFRGIVHAGRSIGRFLTG (MW 2492). The shown structures were determined by solid state NMR in 4:1 POPC/cholesterol bilayers at P/L = 1:4058. The corresponding Protein Data Bank IDs are 6PF0 (P1) and 6PEZ (P3). The α-helices of the peptides lay at the bilayer-water interface, adopting orientations that are almost parallel to the bilayer surface. (C) Cartoon describing the tethered bilayer membrane used for Surface Plasmon Resonance (SPR), Electrical Impedance Spectroscopy (EIS) and neutron reflectometry (NR). The tethered molecules (green) create a 10 Å thick sub-membrane aqueous space. (D) The simplest electric circuit model to describe the surface-supported bilayer, characterized by the membrane capacitance (Cm), resistance (Rm), and solvent resistance (Rsol). (E) Oriented lipid multilayers with peptide incorporated as described in the Methods for the neutron diffraction (ND) experiments. The repeat spacing (d) denotes the dimension of the repeat unit (thickness of the bilayer with its hydration layer).