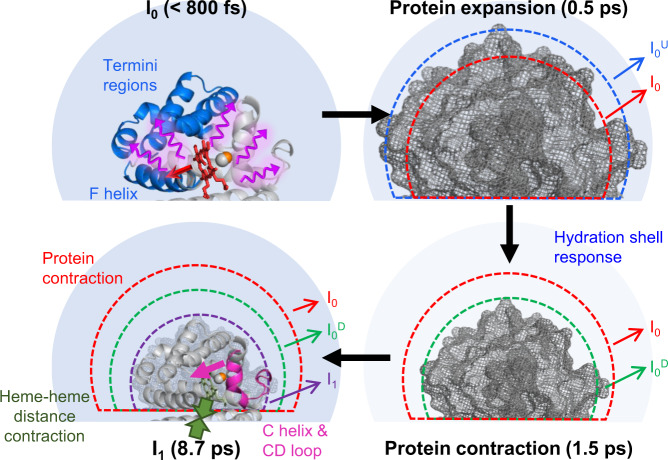
Fig. 5. Schematic for the initial response of HbI(CO)2 upon photoexcitation.
(Left upper panel) The photodissociation of carbon monoxide induces the structural change of the nearby helices such as the F helix that binds the heme group. The structural deformation in these helices is transmitted to C- and N-terminus regions within ~800 fs. (Right upper and right lower panels) In the early time domain, the protein undergoes a non-thermal coherent motion, which leads to the expansion and contraction of the protein similar to a breathing motion. The electron density of the hydration shell, which is indicated by the clarity of the color in the light blue circle around the protein, decreases up to ~1.5 ps after photoexcitation. (Left lower panel) Then the thermal motion of helices leads to the transformation of I0 to I1, accompanying the movement of the C helix and CD loop toward the heme group, the contraction of volume, the shortening of the heme–heme distance, and the change of the electron density of the hydration shell.