Abstract
Tumour recurrence is a serious impediment to cancer treatment, but the mechanisms involved are poorly understood. The most frequently used anti-tumour therapies—chemotherapy and radiotherapy—target highly proliferative cancer cells. However non- or slow-proliferative dormant cancer cells can persist after treatment, eventually causing tumour relapse. Whereas the reversible growth arrest mechanism allows quiescent cells to re-enter the cell cycle, senescent cells are largely thought to be irreversibly arrested, and may instead contribute to tumour growth and relapse through paracrine signalling mechanisms. Thus, due to the differences in their growth arrest mechanism, metabolic features, plasticity and adaptation to their respective tumour microenvironment, dormant-senescent and -quiescent cancer cells could have different but complementary roles in fuelling tumour growth. In this review article, we discuss the implication of dormant cancer cells in tumour relapse and the need to understand how quiescent and senescent cells, respectively, may play a part in this process.
Subject terms: Cancer therapeutic resistance, Cancer microenvironment
In this review article, Karla Santos-de-Frutos and Nabil Djouder discuss the implication of dormant cancer cells in tumour relapse and the roles quiescent and senescent cells may play in this process.
Introduction
Despite our growing knowledge of tumour biology and genetics, cancer remains a deadly disease. A high percentage of treated patients relapse after surgery or adjuvant therapies, and the tumour cells involved in the relapse often exhibit increased tumour propagating potential, manifested as local or distant disease recurrence. However, the mechanisms of tumour recurrence are largely unknown.
In addition to their genetic modifications, tumours comprise heterogeneous masses of cells that may differ in their capacity to support tumour growth, metastasis or resistance to therapy1. A growing tumour mass may consist of millions of proliferating cells, but also of some non- or slow-proliferative cells that are not sensitive to anti-proliferative therapies. Resistant dormant cells could fuel tumour regrowth after disease remission. However, our knowledge of the biology of dormant tumour cells is cripplingly limited. The recent identification of therapy-resistant cell populations with dormancy potential in both solid and hematologic tumours, including melanoma2, glioblastoma3, leukaemia4 and pancreatic5,6 and ovarian7 cancers suggests that these dormant populations, resistant to cancer treatments, play a role in tumour relapse. Furthermore, dormant quiescent cancer cells, also referred as slow-proliferating or slow-cycling cancer cells throughout this review—which stall in G0 phase or rarely enter the cell cycle, and/or senescent cancer cells in tumours could contribute to therapy resistance and tumour recurrence (Fig. 1)2,8. However, solid in vivo evidence of persistent tumour cells involved in tumour relapse are lacking and the molecular mechanisms behind such recurrence are largely unknown. Development of new genetic mouse models to track dormant cells would help to better understand how dormancy could fuel tumour relapse.
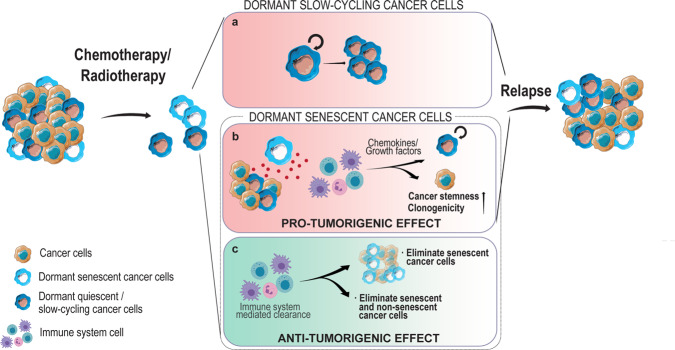
Fig. 1. Schematic representation of the hypothesis of the intratumoural heterogeneity and the effects of anti-proliferative therapies.
Most frequently used anti-proliferative therapies are meant to eliminate rapid-proliferative cancer cells. The remaining dormant cell-driven relapse mechanisms differ depending on whether the cells involved are quiescent/slow-cycling or senescent. a Dormant-quiescent/slow-cycling cells can re-enter the cell cycle in response to appropriate microenvironment changes or SASP signals secreted by senescent cells. Dormant senescent cancer cells can have pro- or anti-tumorigenic effects mainly depending on the SASP content and hence, recruiting immune cells. b Although dormant-senescent cells have apparently undergone irreversible growth arrest, their SASP secretion induces slow-cycling cell proliferation, mainly mediated by immune cell recruitment, and induces clonogenicity and cancer stemness in neighbouring cells. c Alternatively, immune system cells recruited by SASP may eliminate senescent cancer cells, or eliminate both senescent and non-senescent cancer cells, causing tumour eradication39.
The tumour-initiating ability of dormant cells, their capacity to self-renew and ability to differentiate into various tumour bulk subpopulations led the scientific community to think about the involvement of cancer stem cells (CSCs) in tumour relapse9. The general feature of CSCs is their ability to initiate tumour outgrowth, and several similarities are shared between the theory of CSCs of tumour development and the concept of cancer dormancy10. CSCs, like dormant cancer cells, survive conventional cancer therapies and can evade anti-tumour immune responses. However, several lines of evidence suggest that CSCs can consist of distinct heterogeneous subpopulations, including fast-cycling or slow-cycling/quiescent subpopulations11. The quiescent subpopulation could be directly linked to dormant cancer cells, and might therefore exploit latency state to ensure long-term tumour maintenance upon critical environments. Thus, CSCs could be considered as quiescent subpopulations critical in the switch from dormancy to proliferation state to promote tumour outgrowth. Based on their similarities, eradication of dormant cells could also be translated into strategies to eliminate slow-cycling CSCs to eventually minimize the risk of cancer relapse. Further insights into the CSC biology could help us to better understand the mechanisms underlying cancer cell dormancy.
Here, we discuss and stress the need to elucidate the roles of dormant quiescent/slow-cycling and senescent cancer cells, which represent a therapy-resistant cell population reservoir in tumour relapse, often occurring after few months and even several years in the absence of appreciable tumour following therapies. Moreover, mechanisms by which this dormant cell population persist and survive cancer treatment will be discussed.
How do dormant cells resist anti-cancer therapies?
Surgery, chemotherapy, and radiotherapy are the most commonly used cancer treatments, despite immune, hormonal, and targeted therapies are becoming more frequently used. Angiogenesis inhibitors, proteasome inhibitors, small-molecule inhibitors (such as tyrosine kinase, mTOR or PARP inhibitors), monoclonal antibodies (such as EGFR or HER2 inhibitors), and drugs that target histone deacetylases or retinoic acid receptors are among the currently used targeted therapies. Cancer chemotherapeutic agents are commonly categorized into cytotoxic and cytostatic drugs, which typically kill both healthy and cancer cells, and genotoxic agents, which directly or indirectly induce DNA lesions and damage12. Radiotherapy uses ionizing radiation, which also directly affects DNA structure by inducing DNA strand breaks, particularly, double-strand breaks13. Chemo- and radiotherapy both target rapidly dividing cells, causing their death.
Despite the existence of many different cancer chemotherapeutic drugs and, targeted and efficient anti-cancer radiotherapy prescribed after surgery, a high percentage of patients experience relapse after months or even years of treatment discontinuation. Depending on the type of cancers, tumour recurrence is generally considered to occur early or late. In the case of acute lymphoblastic leukaemia most studies classify early tumour relapse as occurring between the first 18–36 months from diagnosis, and late relapse those as occurring after 36 months14,15. On the other hand, breast cancer recurrence is categorized as early when tumour recurrence occurs before 5 years of diagnosis and late after 5 years’ time16,17. In general terms, early recurrence is more prone to occur, while late relapse is thought to be developed due to a long-term dormancy. The probability that a tumour relapse clearly depends on the cancer type. For instance, 20–40% of breast cancer patients18 and 50–70% of hepatocellular carcinoma (HCC) patients19 develop recurrence over a 5-year period, and relapse is almost inevitable in glioblastoma patients20. Why does this occur? Since most chemotherapeutic agents and radiotherapy treatments are designed to eliminate rapidly proliferating cancer cells, tumour relapse may be driven by non- or slow-cycling dormant resistant cells within the tumour that give rise to tumour dormancy21 (Fig. 1). For example, in the case of radiotherapy, glioma cells resistant to ionizing radiations reportedly display increased DNA damage-response mechanisms, inducing therapy refractivity22. Moreover, recent in vitro studies mimicking aromatase inhibitor-induced resistance have identified a so-called pre-adapted cell population which triggers a dormant or sleeper state resistant to therapy, facilitating tumour relapse23.
Different consensual models have been proposed to explain the survival of residual or dormant cancer cells, most of which are based on pre-exiting rare sub-clones that carry mutations conferring resistance to therapies. However, numerous recent studies are elevating the importance of non-mutational mechanisms and propose that mutation-induced resistance could not be the main mechanism leading to dormancy24,25. Interestingly, observations suggest that dormancy can be an adaptive strategy for cancers during times of stress26 and in cases where undetectable residual cancer cells make the patient asymptomatic. Recent studies based on cellular barcoding on colorectal and breast cancer cells suggest that dormant cells are not a pre-exiting population in tumours, but that cancer cells have an equipotent ability to enter the dormant state, similar to the embryonic diapause24,25. The origin of dormant cancer cells in tumour relapse remain elusive; whether these dormant cells pre-exist in the tumour and chemotherapy promotes their selection, or cancer therapies induce their transition to a dormant state in a subpopulation of cancer cells still needs to be confirmed.
Quiescent cells vs. senescent cells
Cellular dormancy is often defined as a non-proliferating state of a cell, but commonly discussed in terms of two growth arrest mechanisms: quiescence, in which cells are in a non-proliferative or slow-cycling state, with a reversible growth arrest, and senescence, in which cell cycle arrest is largely irreversible10,27,28. The mechanisms of tumour relapse induced by reactivation of dormant cancer cells depend on whether the cells became dormant via quiescence or senescence.
Dormant quiescent slow-cycling cancer cells
As noted above, quiescence is considered a reversible state in which a cell ceases to divide but retains the ability to re-enter the cell cycle. It is generally believed that quiescence is the most appropriate mechanism for describing cellular dormancy10,27. In particular, dormancy has been demonstrated to represent a special case of quiescence among stem cells29. Quiescence is a cellular process that preserves stem cell function in case it is needed in tissue homeostasis or repair, and shares feature with senescent cells30,31. Such dormant quiescent cells are also known as slow-cycling or slow-proliferating cells because they stall in G0-G1 phase or rarely enter the cell cycle. Quiescent cells are arrested in the G0-G1 phase, meaning that the activity of cyclin-dependent kinases (CDK) is reduced, while the activity of the CDK inhibitor p27, which regulates the transition from G0 through G1 into S phase, is elevated32,33. In response to injury, quiescent stem cells transit between the G0 phase and an ‘alert’ phase called G(Alert), a process controlled by mTORC1. G(Alert) represents an adaptive mechanism to respond rapidly to damaging reagents and stress, priming stem cells for a rapid cell cycle entry to repair the injured organ34. Interestingly, the G0 phase is characterised by low metabolic activity, with a decrease in the production of ribosomal RNA and proteins, leading to reduction of their volume and size35. Recent studies have suggested that quiescent cells could have an embryonic diapause-like state in breast and colorectal cancers. This diapause-like state is defined by decreased mTOR activity, leading to increased autophagy, suggesting that chemotherapy combined with autophagy inhibitors could be efficient to kill these quiescent cancer cells24,25. However, a deeper analysis of their transcriptomic signature demonstrates that this quiescent cancer cells might be distinct from the diapause state described for embryos, but rather resemble the paused embryonic stem cells24. Clearly, quiescent cells might adopt different states of dormancy which should be further characterized by developing genetic tools allowing their labelling and track in vivo during tumour recurrence, and by single-cell RNA-sequencing methodology.
Whereas highly proliferative cells promote DNA replication stress-driven mutations, the quiescent state seems to enable cancer cells to acquire new somatic mutations essential for disease progression. In fact, quiescent cells express the lower levels of genes involves in DNA damage repair mechanisms36. Moreover, the preferential use of the more error prone non-homologous end joining-mediated DNA repair mechanism rather than homologous recombination renders quiescent cells more susceptible to suffer genomic instability and transformation upon DNA damage37. These new mutations might facilitate quiescent cancer cells to escape the immune system. Agudo et al. demonstrated that slow-cycling cells are immune-protective, a mechanism that is not specifically shared by rapid proliferating cells11. Furthermore, several studies on quiescent cells have suggested that immune evasion could be obtained through neo-antigen loss. Recent single-cell RNA-sequencing analysis on HCC suggested that the loss of main clonal neo-antigens during relapse could explain the impossibility of CD8+ T cells to recognize cancer cells and induce their clearance38. Moreover, new mutations in quiescent cells may lead to new sub-clonal neo-antigens, escaping the memory T cells.
Natural killer (NK) cells are known to be implicated in tumour cell clearance by the secretion of several inflammatory cytokines, eliminating quiescent and senescent cancer cells. Iannello et al. demonstrated that NK cells are recruited by the secretory phenotype of senescent cells mediated by p5339. An example of immune evasion involves the cell surface glycoprotein UL16 binding protein 1 (ULBP1), a member of the MHC class I superfamily, which is expressed on the surface of malignant transformed cells40. ULBP1 functions as a stress-induced ligand for NKG2D receptor, activating NK cell-mediated cytotoxicity40–42. Recent studies in breast cancer and lung adenocarcinoma suggest that slow-cycling or quiescent cells downregulate ULBP ligands (ULBP1-5), thereby inactivating NK cells and allowing the CSCs to repopulate the tumour niche43. The authors demonstrated that expression of DKK1, an autocrine WNT inhibitor, results in the downregulation of NK activating ligands and death signal receptors. DKK1 depletion leads to NK cell-mediated cytotoxicity of quiescent cells in vitro, but the authors did not show that ULBP ligand is critical for NK evasion of quiescent cells. The reversibility of growth arrest in quiescent cells may thus enable slow-cycling cancer cell-mediated tumour relapse in cases where microenvironmental changes enable these dormant cells to resume normal cell cycle behaviour (Fig. 1).
Several studies have focused on deciphering the involvement of quiescent cells in tumour relapse. It was proposed that recurrence of basal cell carcinoma after vismodegib treatment was due to a switch of proliferative Lgr5-expressing cells to quiescent cells that become non-targeted by vismodegib, leading to tumour relapse21. Another study reached the same conclusions and proposed a similar phenotypic switch in basal cell carcinoma leading to tumour relapse. The authors demonstrated that quiescent cells were able to re-enter a proliferative state after vismodegib discontinuation promoting tumour regrowth44. Furthermore, a rare quiescent cancer stem cell pool was identified in squamous cell carcinoma that becomes enriched following 5-FU treatment and displays increased tumour propagating potential. The quiescent stem cell pool co-existed with proliferative cells and transcriptomic analysis suggested that the dynamic transition between quiescent and proliferative states was mainly controlled by pro- and anti-proliferative cancer signalling factors, such as TGF-β. Further, TGF-β was the crucial factor directing quiescence in squamous cell carcinoma45. More studies are clearly needed to understand how slow-cycling cells escape from the immune system and re-enter in the cell cycle to promote tumour relapse. Moreover, despite the studies suggesting that tumour relapse is due to slow-cycling cells which persist after cancer treatment, further work is needed to fully demonstrate that tumour recurrence indeed relies on non-targeted quiescent cancer cells. Animal models to track these cells during tumour recurrence are urgently required to demonstrate the role of slow-cycling cells in tumour relapse. Moreover, the presence of senescent cells resistant to the mentioned therapeutic agents and their possible implication in recurrence21,44,45 cannot be excluded and will be discussed below.
Dormant senescent cancer cells
By contrast, senescent cells are irreversibly arrested in the G1–G1/S phase46,47. The cellular senescence programme can be activated by a wide range of extrinsic and intrinsic stressors, which eventually lead to activation or expression of the tumour suppressors p53 and/or p16INK4A48–51. Serrano et al. showed that Ras-mediated senescence requires p53 and p16INK4A/Rb to promote cell cycle arrest49. Telomere damage, oxidative stress and DNA damage, among others, activate p53, which induces p21 expression to inhibit the cyclin E-Cdk2 and promote cell-cycle arrest52,53. p16INK4A, in contrast, which can be activated by various oncogenes, epigenetic stress or nucleolar stress, inhibits cell-cycle progression via the disruption of the cyclin D-Cdk4/6 complexes48,52,54. p53 or p16INK4A-related pathways impede RB phosphorylation and hence, its inactivation. In turn, this leads to inhibitory binding to E2Fs transcription factors, thus preventing the expression of genes involved in cell proliferation and DNA replication49,55. Senescent cells are also metabolically very active, displaying an increased biomass. This high activity is needed to secrete stress-mediated granules51,56. Accordingly, senescence-mediated lysosomal compartment expansion leads to an increase SA-β-galactosidase or β-D-galactosidase activity57, commonly used as a senescence biomarker58. Moreover, senescent cells exhibit an altered chromatin structure called senescence-associated heterochromatin foci (SAHF) which stains densely with DAPI and is enriched for histone modifications, mainly lysine 9-trimethylated histone H3. SAHFs play a role in the senescence-associated cell growth by sequestering and silencing proliferation-promoting genes, including the E2F target gene cyclin A55.
Cellular senescence is thus a state of permanent cellular growth arrest induced by damage or stress. The detection of senescence markers in dormant cancer cells suggested that senescence may be another mechanism driving cellular dormancy. This idea is supported by two studies59,60, reporting that BMP7 and SPARC, respectively, maintain prostate cancer cells in dormancy by inducing senescence59,60. The authors showed that when culturing metastatic prostate cancer cell lines in the presence of conditioned media from human bone marrow stromal cells, senescence-associated markers were upregulated. Particularly, they demonstrated in vitro that bone stromal cell-secreted BMP7 induces senescence by activating p38 MAPK signalling, in turn increasing the level of p21, which mediates the upregulation of the metastasis suppressor gene NDRG1 expression, ultimately resulting in cell-cycle arrest or dormancy59. p38 is known to be involved in cell cycle arrest regulation and plays a crucial role in the induction of senescence in response to a variety of stresses61.
Despite senescence being considered a state of irreversible growth arrest, it is estimated that 1 in 106 senescent cells could escape from senescence and re-enter the cell cycle62. Studies in non-small cell lung cancer cell lines suggest that chemotherapy-induced senescent arrest can be reversible in a small subset of cells, which mainly escape through the upregulation of Cdk2/Cdk1. The authors showed that 3–4 weeks after removal of the chemotherapeutic drug camptothecin, some cells were able to form colonies62. Furthermore, SPARC, a matrix-associated protein expressed and secreted by prostate cancer cells, induces dormancy of bone cells, a process sustained by SPARC-mediated activation of BMP7 secretion. Depletion of SPARC reawakens these dormant cells, leading to their growth60. Interestingly, Milanovic et al. demonstrated that a rare fraction of senescent cells could spontaneously be released from senescence and re-enter the cell cycle, giving rise to the so-called “post-senescence” state. The authors suggest that these “post-senescent” cells retain stem cell-related features (also known as senescence-associated stemness), suggesting a more aggressive behaviour and favouring tumour relapse30. Yet, senescence reversibility seems an infrequent event. In support of this idea, Takahashi et al. suggested that blockage in cytokinesis could be a second barrier for cellular senescence, where p16INK4a-Rb pathway and senescence-associated chromatin remodelling support the irreversible cellular arrest, limiting senescence plasticity and implying the infrequency of this event63.
The general properties of senescent cells may suggest that their main role in tumour recurrence does not involve reinstating the cell cycle. Instead, it is more likely to be driven by the release of secretory factors from senescent cells, which may modulate the microenvironment and particularly the behaviour of nearby immune cells. Immune system modulation is mainly driven by cytokines, chemokines, matrix remodelling proteases, and growth factors secreted by senescent cells exhibiting the so-called senescence-associated secretory phenotype (SASP)8,64–66. DNA damage leads to SASP programme activation, which is carried out by stress-response kinases. SASP or growth factors secreted by senescent cells could activate slow-cycling cells’ proliferation in a paracrine way and/or via immune system activation, leading to tumour relapse (Fig. 1). In addition to the presence of cancer cells and stroma, innate (such as macrophages, neutrophils and NK cells) and adaptive immune cells (T and B lymphocytes) form part of the tumour microenvironment67. All these cell types communicate via autocrine and paracrine signals mediated by several immune modulators, such as chemokines and cytokines. The cellular diversity within the same inflammatory niche, the activation states of these various cell types, as well as the class and expression levels of the immune modulators will determine the pro- or anti-tumorigenic effects of the SASP68. Depending on this response, SASP can lead to the clearance or the protection of cancer cells, favouring cell dormancy and tumour recurrence. For instance, various physiological processes, such as increased cell survival, angiogenesis and suppression of anti-tumour adaptive immune responses are regulated by leucocyte infiltrates67. Moreover, the transcription factor NF-κB, a key mediator of inflammatory responses, regulates the expression of genes involved in the suppression of tumour cancer cell death, activates tumour cell cycle progression and stimulates epithelial-to-mesenchymal transition and angiogenesis69. SASP factors could therefore activate the pro-tumorigenic inflammatory response and thus, via the activation of inflammatory cells, promote surrounding cells’ (slow-cycling cells) to proliferate. This crosstalk between senescent cells and slow-cycling cells enables the latter to become highly proliferative following paracrine signal activation. Consequently, senescent cells retain tumour propagation potential and can drive tumour re-initiation after chemo or radiotherapy. Pre-malignant senescent hepatocytes were found to accelerate the growth of HCC cancer cells in mice and humans mainly through SASP secretion-mediated immune recruitment70,71. Further, senescent cell-secreted IL-6 promotes reprogramming of the surrounding cells in vivo72 and conditioning with senescent cell media promotes clonogenicity and cancer stemness in multiple myeloma cell lines73, and to enrich chemotherapy-resistant cell populations in vitro in malignant pleural mesothelioma cell lines74.
The presence of senescent cells may also favour other physiological processes, such as wound healing31, embryonic development75,76 and maturation of β cells77. On the other hand, cellular senescence contributes to non-cancerous pathologies. The accumulation of aberrant senescent cells generates an inflammatory niche, which might induce tissue damage and the development of various diseases, such as liver and lung fibrosis, diabetes, atherosclerosis and osteoarthritis46,78,79. Interestingly, the elimination of senescent cells improves these pathologies and contributes to longevity46,78,80–82. Liver fibrosis can be an example of the role played by senescence in disease progression, which clearly depends on the cell type undergoing senescence and the inflammatory milieu generated. The general idea is that senescent hepatocytes and cholangiocytes are associated with fibrosis progression, most likely through paracrine signals activating hepatic stellate cells (HSCs) which are implicated in the production of the extracellular matrix of fibrotic scars83. However, senescence of HSCs can induce fibrosis regression by enhancing the expression of the matrix metalloproteases with fibrolytic activity, enabling the tissue to recover, and hence limiting liver fibrosis84. Moreover, senescent HSCs can modulate an immuno- surveillance response to promote their clearance via the activation of the NK cells, leading to the resolution of fibrosis84.
The abovementioned findings clearly support the idea that quiescence and senescence are associated with different forms of dormancy that lead to distinct phenotypes capable of driving tumour relapse (Table 1). This complexity reinforces the necessity to better elucidate the mechanisms by which slow-cycling and senescent cancer cell populations participate in tumour relapse.
Table 1.
Main differences between dormant senescent and quiescent cancer cells and their roles in tumour relapse.
| Quiescent cancer cell | Senescent cancer cell | References | |
|---|---|---|---|
| Cell cycle arrest | Reversible: G0-G1 phase arrest | Irreversible: G1-G1 / S phase arrest / Cytokinetic block | 32,33,46,47,63,139 |
| Markers | None |
• p16INK4 expression / p53 activity • SASP factors • SA-β-gal staining • DNA damage-response • γH2AX foci and SAHF formation |
46,49,58 |
| Effectors | p27 | p53 (and p21) and / or p16INK4 mediated RB activation | 49–51 |
| Metabolic activity | Low (reduction in volume and size) | Very active (increased biomass leading to SASP production) | 33,51,56 |
| Role of immune system | Immune evasion | Attract immune cells by SASP secretion | 8,43,65,70 |
| Mechanisms of relapse | Re-enter cell cycle | Microenvironment modulation and immune cell recruitment via SASP | 21,30,44,68,70,100,101 |
| Structural changes | Chromatin compactation by methylation in H4K20 | SAHF formation and γH2AX foci / Lysosomal compartment expansion | 55,57,140 |
RB retinoblastoma protein, SA-β-gal senescence-associated β-galactosidase, SAHF senescence-associated heterochromatic foci, SASP senescence-associated secretory phenotype.
The duality of senescent cells: anti- or pro-tumorigenic?
Senescence is considered a stress response induced by several intrinsic and/or extrinsic factors and mechanisms, such as chemotherapeutic agents, hypoxia, oncogene activation, aging and dysregulation of growth factors. Various studies have suggested that classical cytotoxic therapies, molecularly targeted therapies and immunotherapies can all trigger so-called “therapy-induced senescence” (TIS), converting tumour cells into senescent cells64. Remarkably, the widely used chemotherapeutic agent doxorubicin, which affects DNA structure, can enlarge the senescent cancer cell pool85. ATRX as a key regulator of TIS and indeed, both DNA-damaging agents, such as chemotherapeutic drugs and CDK4 inhibitors require ATRX expression and subsequent suppression of the HRAS locus to promote senescence induction. ATRX-depleted cell lines enter quiescence, following treatment with chemotherapeutic agents and CDK4 inhibitors86.
Whereas the literature has mainly focused on chemotherapy-induced senescence, radiotherapy can also induce senescence in cancer cells87–89. TIS can have a profound impact, particularly in fractionated radiotherapy regimens where the radiation dose is increased incrementally. Because each dose of ionizing radiation will convert some tumour cells into senescent cells, the treatment may not have the expected anti-tumour effect by the time the patient receives the highest doses. Unlike in apoptosis, cells that enter senescence are not killed; they remain in the tumour and retain metabolic and secretory activity despite not undergoing cell division90. Moderate doses of camptothecin convert 85–90% of non-small cell lung adenocarcinoma cell lines to senescent cells, while etoposide induces 40–60% and cisplatin 10–30% of cells to enter senescence. These senescent cells are identified by flattened morphology, increased cytoplasmic granularity, SA-β-galactosidase expression and reduced proliferation62. Furthermore, other studies of chemotherapy-treated breast cancer patients tumour samples revealed that 41% of treated samples were positive to SA-β-gal91. Hence, although, TIS can also be detected in treated patients, senescent cells may have either pro- or anti-tumorigenic effects depending on their cellular or pathophysiological context and their production of secretory factors or SASP, which, as discussed further below, have pleotropic functions and is a two-edged sword in cancer8,28,64,65
Anti-tumorigenic effects of senescent cells
Not only can senescence-associated cell cycle arrest inhibit tumour growth and progression8,64, but the associated SASP can also modulate and reshape the tumour microenvironment to stimulate immune-mediated clearance of senescent cancer cells. SASP factors have different biological activities, and dynamic SASP patterns have been observed. The senescence process appears to have at least two distinct secretory phases in which different subsets of factors are secreted with opposite effects. The “first wave” or phase is mediated by cell-to-cell contact (juxtracrine) between senescent and neighbouring cells via the activation of NOTCH, and which leads to cell-intrinsic and extrinsic effects. NOTCH signalling pathway relies on ligand-dependent activation (JAG1/2 and DLL1/3/4 in humans) and it has to undergo a series of proteolytic cleavage steps, leading to the formation of NOTCH intracellular domain (NICD). NICD can translocate to the nucleus, where it induces the transcription of NOTCH target genes, thereby promoting “lateral senescence” or “paracrine senescence” of neighbouring cells92. In this regard, NOTCH modulates the expression of inflammatory cytokines, including the critical SASP factor TGF-β, which reinforces the paracrine senescence through p21-mediated cell cycle arrest93. Likewise, the transmission of senescence to neighbouring cells via a paracrine signal sets-up a tumour-suppressive function94,95. The “second wave” secretome is usually rich in C/EBP-β-dependent SASP with pro-inflammatory, fibrolytic and immune clearance properties. C/EBP-β induces the expression of inflammatory cytokines, such as IL-6 and IL-8, which attract and activate a wide range of immune system cells (e.g. CD8+ cytotoxic T-cells, B-cells, neutrophils)96, favouring immune-mediated elimination of senescent cells65. The existence of a coordinated response of innate immune components required for the clearance of senescent cancer cells has been suggested94,95,97. A study of hepatocarcinoma provided an example of this type of anti-tumorigenic senescence, demonstrating that p53 loss is required to maintain the aggressiveness of cancer cells and its restoration induces senescence, immune recruitment and tumour cell clearance94. In addition, immune-mediated clearance of pre-malignant senescent hepatocytes is mainly driven by CD4+ T-cell based adaptive immunity by the secretion of diverse chemo- and cytokines, which also requires the activation of monocytes and macrophages95. Moreover, senescence-associated cell cycle arrest can inhibit tumour growth and progression8,64. Studies in KRAS mutant models of lung cancer demonstrated that the combination of MEK and CDK4/6 inhibitors lead to TIS, whereas components of SASP attracted NK cells, contributing to tumour regression98. The authors extrapolated their findings to poorly vascularized pancreatic ductal adenocarcinoma, and demonstrated that combinatory targeted therapies triggered senescence and in turn, SASP remodelled the tumour microenvironment and vascularity, increasing blood vessel density and permeability in order to facilitate chemotherapeutic agent uptake within the tumours and increased T cell infiltration, rendering it susceptible to immune checkpoint inhibitors99. Despite these findings suggesting that senescence can support tumour-suppressive mechanisms to restrict the development of malignant cells, they do not exclude that after a certain time, these senescent cells could negatively remodel the tumour microenvironment to favour tumour relapse once treatment is ceased.
Pro-tumorigenic effects of senescent cells
In some cases, SASP can also inflame the tumour microenvironment and accelerate tumour progression8,28,64,65, probably depending on the SASP as well as on the type of immune cells composing the inflammatory milieu, which could influence proliferation and growth of cancer cells or activate the invasive properties of cancer cells including migration and angiogenesis30,100. SASP could also impair the immunosurveillance response by inhibiting the immune-mediated clearance, thus enabling cancer recurrence70. This ability of senescent cells to modify the microenvironment and the surrounding cells in a non-autonomous manner adds further complexity to tumours. Studies on several types of cancer have suggested that cellular senescence and SASP are barriers to complete tumour eradication, even though senescence has often been regarded as an intrinsic tumour suppressor mechanism like apoptosis8,64–66. Krtolica et al. were among the first to suggest that senescence may exhibit evolutionary antagonistic pleiotropy, which means that can have both beneficial and deleterious effects. They showed that soluble and insoluble factors secreted by senescent fibroblasts caused pre-malignant and malignant epithelial cells to proliferate and form tumours100. Furthermore, in a liver cancer mouse model, myeloid cells recruited by SASP factors released from pre-malignant senescent hepatocytes created a pro-tumorigenic and immunosuppressive environment70,101. CCL2, a cytokine, was identified as a key factor secreted by precancerous senescent hepatocytes, favouring the recruitment of CCR2+ immature myeloid cells (iMC). The differentiation and maturation of iMCs to macrophages is essential for precancerous senescent cell clearance. In contrast, iMC accumulation led to HCC through the inactivation of the NK cell function. Interestingly, Eggert et al. showed that tumour cells prevented the maturation of iMC to macrophages through SASP secretion, which in turn resulted in tumour immune escape70. However, the pro- and anti-tumorigenic profiles of SASP are poorly defined and difficult to predict in the context of tumour relapse. How SASP modulates these opposing effects depending on the microenvironment or pathophysiological context remains to be determined. Moreover, it is not excluded that various senescent states might co-exist to shape the tumour microenvironment and modulate the pro- or anti-tumorigenic effects. Single-cell RNA sequencing could determine the senescent state phenotypes existing within a tumour.
Strategies to eliminate dormant cells
Strategies to target dormant quiescent cancer cells
Because current therapies target proliferating tumour cells, an important question is which therapeutic approach would be best for eliminating dormant cancer cells. Here, we discuss three different strategies to target dormant quiescent cells (Fig. 2): “awakening” or enhancing the proliferation of dormant slow-cycling resistant cancer cells to increase their susceptibility to anti-proliferative drugs, keeping cells in a dormant state, and eradicating them while dormant-quiescent or slow-proliferating.
Fig. 2. Schematic representation of the different strategies to eradicate dormant quiescent cancer cells.
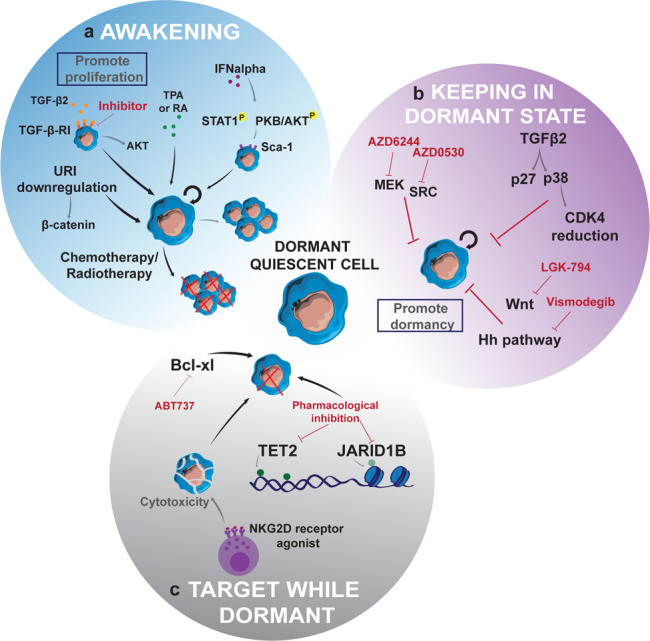
Dormant quiescent or slow-cycling cells can mainly be targeted by three different strategies: a awakening, which aims to promote re-enter in cell cycle and proliferation of quiescent cells in order to be correctly targeted and eliminated by anti-proliferative therapies; b keeping the dormant state to avoid awakening and tumour relapse; and c targeting while dormant, which is based in targeting the crucial signalling pathways needed to keep cells in dormancy, such as epigenetic changes for example.
“Awakening” of dormant quiescent cells
Reactivating dormant quiescent cancer cells to make them rapidly re-enter the cell cycle is expected to improve their elimination by anti-proliferative drugs. When anti-proliferative chemotherapeutic agent resistant hematopoietic stem cells were pre-treated with IFNα, STAT1 and PKB/AKT were phosphorylated, increasing the expression of cell surface stem cell antigen-1, thereby inducing cell proliferation and efficient elimination by 5-fluorouracil (5-FU) in vivo102. In leukaemia, combined treatment with granulocyte colony-stimulating factor (G-CSF) and the cell-cycle dependant chemotherapeutic cytarabine enhanced the proliferation and elimination of quiescent stem cells in acute myeloid leukaemia mouse models103. However, G-CSF treatment followed by chemotherapies including cytarabine and mitoxantrone, or cytarabine, daunorubicin and thioguanine did not improve AML patients’ outcome104. These results highlight the difficulties to translate findings from mice to humans, and point that strategies to awaken quiescent cells are not always easy to apply in patients.
The identification of essential pathways required to maintain a low proliferation rate or a dormant state could facilitate the design of effective “awakening” treatments that could be combined with anti-proliferative therapies to prevent cancer relapse. In line with this strategy, downregulation of the molecular chaperone URI (unconventional prefoldin RPB5 interactor) in intestinal label-retaining slow-cycling cells induced β-catenin expression and made cells highly proliferative and radiosensitive105. Reducing URI levels could thus be one way to increase the proliferation rate of slow-cycling cancer cells, making them more sensitive to chemo- or radiotherapy. Moreover, when proliferation of vismodegib-resistant quiescent cells was reinstated with retinoic acid or 12-O-tetradecanoylphorbol-13-acetate in basal cell carcinoma, remaining dormant cancer cells were completely eliminated by vismodegib, abolishing tumour relapse21. Therefore, reactivation of dormant cells into a proliferative stage followed by radio or chemotherapy could be an efficient therapeutic strategy against tumour relapse. Despite several studies suggesting that dormant cell reactivation as part of an “awakening” strategy could overcome chemotherapeutic drug resistance, the clinical implementation of this strategy is likely to be challenging because it is difficult to ensure that all cells will re-enter the cell cycle and then be eliminated. Indeed, recent studies suggest that such strategies could rapidly fuel tumour recurrence and worsen patient outcomes in some cases because of these remaining dormant quiescent cells. For instance, TGF-β2 was identified as a crucial inductor of dormancy in head and neck cancer cell lines. Inhibition of TGF-β receptor with LY-364947 in mouse models resulted in reactivation of dormant cells and an increase in metastatic burden in liver, spleen and bone marrow106.
Keeping cells in a quiescent state
Another strategy to prevent tumour relapse involves maintaining dormancy to avoid rapid proliferation and tumour regrowth. Recent studies have revealed cues that promote cellular dormancy, which could enable the development of therapies that mimic the pro-dormancy mechanisms and thereby prevent tumour recurrence. Dormant cells are characterized by increased p38 MAPK and decreased ERK1/2 activities, which are widely used as dormancy markers107. Despite being active in senescent cells, strategies to modulate the p38/ERK pathways could lead to permanent growth arrest of quiescent cells, preventing tumour recurrence and metastasis108–110. Moreover, pharmacological inhibition of SRC and MEK could prevent the proliferative response of dormant quiescent cells to external stimuli and suppress their survival in breast cancer, preventing its recurrence111. Likewise, because dormant quiescent cancer cell awakening is thought to be the last step in metastatic outbreaks, blocking factors involved in this process could be a powerful and precise way of preventing metastasis108. In addition, activation of Wnt signalling was reported to be implicated in the switch from vismodegib-resistant quiescent cells to proliferative cancer cells, and a combination of inhibitors against Wnt and hedgehog pathways abolished relapse of basal cell carcinoma21,44. Several previous studies have proposed the crucial role of the extracellular matrix components in quiescent cell awakening, particularly the β1 integrin signalling pathway112,113. Neutralizing antibody-mediated β1 integrin blockage leads to MLC phosphorylation, loss of actin stress fiber formation and prevents the switch of quiescent breast cancer cells to proliferative status113. Moreover, microenvironment-induced TGFβ2 signalling activates p27 and downregulates CDK4 via p38α/β, leading to cell dormancy106 and activation of p38 induces p53 and BHLHB3 expression while inhibiting that of c-Jun and FoxM1114.
Since the ability to switch from quiescence to proliferative state could be an issue for slow-cycling cells, keeping them in a quiescent state would be the best approach to prevent tumour recurrence. However, despite the aforementioned promising results, the proposed strategy requires the dormant state to be preserved for a long time to prevent tumour regrowth, which may be very difficult to achieve given the high adaptability of cancer cells to different scenarios. Furthermore, the strategies to maintain cells in dormancy for a long period requires a permanent treatment, which seems clinically unviable, mainly due to toxicity. Furthermore, long-term treatments could always give rise to resistance, causing more complexities in dealing with tumour relapse.
It is worth mentioning that Salvador-Barbero et al. suggest that treatment with CDK4/6 inhibitors to prevent cell cycle entry after treatment with antimitotic or DNA-damaging chemotherapeutics might improve pancreatic adenocarcinoma recovery115. Despite these attempts to target therapy-induced proliferative cancer cells, it remains to be seen in humans whether cell-cycle inhibitors can be used sequentially to efficiently target slow-proliferative cells. Moreover, it should be noted that some senescent cancer cells will remain in the tumour and could thus still rewire the microenvironment to promote recurrence.
Targeting cells while quiescent
Quiescent cancer cells have different characteristics than proliferating cells, opening alternative strategies to eradicate cancer cells in their dormant state. Insulin-like growth factor 1 (IGF-1)/IGF-1 receptor (IGF-1R) autocrine signalling and the subsequent AKT activation was identified as a common mechanism to promote dormancy in KRAS- and c-MYC null-pancreatic cancer cells; and pancreatic dormant cells were eliminated when treated with IGF-1R inhibitor116. In addition, quiescent slow-cycling cells display constant expression of Bcl-xl essential for their survival and inhibition of Bcl-xl by ABT-737 resulted in the elimination of quiescent slow-cycling non-small cell lung cancer cells, highlighting the potential therapeutic use of ABT-737 to eradicate slow-cycling cells117. Likewise, quiescent persistent cancer cells were shown to be sensitive to ferroptosis, a programmed cell death induced by lipid peroxides accumulation. The phospholipid glutathione peroxidase GPX4 protects against membrane lipid peroxidation and in turn, prevents ferroptotic cell death118. GPX4 inhibitor RSL3 selectively reduced the residual persistent cell pool in several types of cancer cell lines including melanoma (A375 cell line), breast (BT474 cell line), lung (PC9 cell line) and ovarian (Kuramochi) cancer cells as well as in A375 melanoma cell lines-derived xenograft models119. Persistent quiescent cells in colorectal cancers could also be eliminated by targeting autophagy. Quiescent cells treated with inhibitors against ULK1, a crucial kinase activating autophagy, in combination with a standard chemotherapy treatment (CPT-11), failed to regrow and underwent apoptosis, even after treatment discontinuation24. Moreover, since quiescent cancer cells evades NK cell recognition by downregulating their stress ligand ULBP1, the use of specific agonists for NKG2D receptor-activating NK cells could lead to the destruction of quiescent cells43.
Unfortunately, the induction of cellular dormancy and retardation of the rate of proliferation appear to be complex processes that may involve robust epigenetic reprogramming, and little is currently known about the epigenetics of slow-cycling cells. The epigenetic enzyme TET2 may be a key factor controlling the numbers and survival of slow-cycling cancer cells as well as tumour recurrence. 5-hydroxymethylcytosine generated by the activity of TET2 was identified as a predictive biomarker of relapse and survival in cancer patients, suggesting that TET2 could be a potential drug target for slow-cycling cell elimination2. In addition, in vitro experiments on melanoma cells showed that both cytotoxic and targeted cancer chemotherapeutic agents caused uniform enrichment of cells expressing the H3K4 demethylase JARID1B. It was therefore postulated that targeting the slow-cycling cell population by inhibiting this enzyme’s demethylase activity while simultaneously applying conventional anti-proliferative therapy could help eradicate all melanoma cells120.
A strategy based on targeting dormant cells would have to be efficient enough to ensure that no slow-cycling/dormant quiescent cells remain. Since no diagnostic tools currently exist to detect dormant quiescent cells in patients, such efficiency will probably be difficult to achieve. Nevertheless, the evidence accumulated to date strongly suggests that persistent or untargeted slow-cycling quiescent cells can become more aggressive and lead to worse prognoses. Identifying unique features and markers of quiescent cells could also allow the development of strategies directing the immune system against dormant cells. As proposed for senescent cells121, developing chimeric antigen receptor (CAR) T cells could be useful to directly recognize and eliminate quiescent dormant cancer cells. These innovative strategies stress out the urgent need to discover surface markers of slow-cycling or quiescent cancer cells. Detecting dormant cells through specific labelling in vivo would help in this task.
Strategies to eradicate senescent cells
As noted above, the SASP can control surrounding cells via paracrine loops. However, because surrounding cells can act as signal relays, it can also indirectly influence the SASP-displaying senescent cells themselves95. Due to their persistent SASP secretion, these cells will be surrounded by radioprotective and chemoprotective factors, as well as growth and angiogenic factors that support tumour progression. It is also known that as the ratio of senescent cells to immune cells increases, senescent cells become more tumour-promoting rather than tumour-suppressive122.
Owing to their molecular complexity and interactions, different strategies to eradicate senescent cells have been proposed (Fig. 3). As senescent cancer cell activity is mainly directed via SASP secretion, SASP modulation for therapeutic purposes could be a promising way of preventing tumour relapse, also known as senomorphic therapy. Various inhibitors have been proposed to induce a switch from pro-tumorigenic SASP to tumour-suppressive SASP. The secretome of senescent cells relies on their genetic background. For instance, senescent cells present in the PTEN-null prostate tumours revealed an immunosuppressive SASP mainly controlled by NF-kB and STAT3 signalling. Treatment of these cells with JAK2/STAT3 inhibitors provoked a SASP secretory switch resulting in an anti-tumorigenic secretome-activating an immunosurveillance response123.
Fig. 3. Schematic representation of the different strategies to eradicate dormant senescent cancer cells.
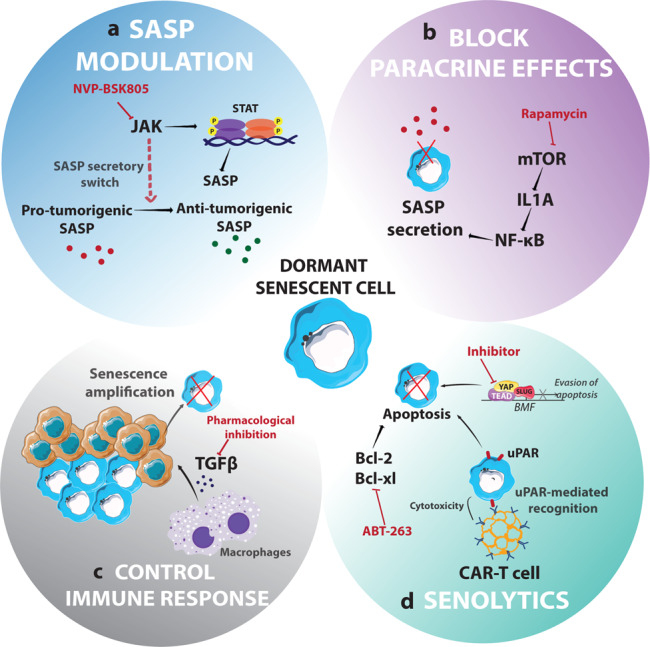
Strategies to eradicate dormant senescent cancer cells have been classified in four groups: since senescent cancer cell activity is mainly directed via SASP secretion, two different approaches can be done a SASP modulation; b blocking the paracrine effects of SASP; c control the immune response in order to avoid senescence amplification; d use senolytic compounds that directly target senescent cells.
Another strategy would be to target senescent cells by blocking the paracrine effects of SASP. Laberge et al. showed that senescence signal-mediated IL-1α translation was mTOR-dependent, and thus rapamycin sensitive. Rapamycin treatment leads to the blockage of IL-1α translation and in turn, reduced NF-κB-mediated SASP factors gene expression activated downstream of IL1R124. Thus, rapamycin suppressed both the establishment and maintenance of SASP, suggesting that rapamycin is a potential viable therapeutic approach to target senescent cells. However, since rapamycin treatment is not exclusive for senescent cells, this therapy might also affect healthy epithelial cells and have deleterious effects125,126. Another example is a study which identified the critical role of the rasGAP SH3-binding protein 1 (G3BP1) in SASP secretion127. G3BP1 depletion in primary human lung fibroblasts induced a “SASPless” phenotype of senescent cells, which were unable to promote tumour growth in vitro and in vivo. Thus, G3BP1 inhibition could block the paracrine effects of senescent cells and in turn, its pro-tumorigenic effect.
Controlling the immune responses to SASP would be an alternative immunotherapeutic approach to ameliorate or promote the anti-tumour activity of crosstalk between SASP and immune cells. SASP-mediated macrophage recruitment leads to macrophage-dependent paracrine TGFβ signalling, which induces senescence amplification in liver injury models. This mechanism could thus be exploited to target TGFβ signalling and thereby reduce the non-cellular autonomous effects of senescence on tumorigenesis128.
Small-molecule agents known as senolytics that selectively target and eliminate dormant senescent cells are becoming increasingly attractive as options for treating cancer and preventing relapse129–131. ABT263, also known as Navitoclax, a well-known Bcl-2 and Bcl-xl anti-apoptotic inhibitor selectively targets and eliminates senescent cells by inducing apoptosis132. Furthermore, a senescent-like dormant phenotype was observed following EGFR/MEK combinatorial treatment in non-small cell lung cancer that enabled tumour recurrence. The authors indicated that YAP/TEAD-mediated epigenetic alterations, via SLUG, a transcription factor of EMT process, suppressed the pro-apoptotic factor BMF, leading to survival of cancer cells133. As YAP/TAZ inactivation had no relevant side effects on the basal homeostasis of surrounding healthy adult tissue134, they proposed that pharmacological inhibition of YAP/TEAD could lead to apoptosis of senescent-like cancer cells, resulting in tumour regression. Very recently, Amor et al. suggested the therapeutic use of CAR T cells as senolytics to target senescent cells. The authors identified urokinase-type plasminogen activator receptor (uPAR) as a cell-surface protein that is induced during senescence and demonstrated that uPAR-specific CAR T cells efficiently depleted senescent cells in vitro and in various disease settings121. Senolytics could be used in combination or sequentially with chemotherapeutic drugs or radiotherapy135. This two-hit anti-cancer strategy would involve first inducing senescence with chemotherapeutic agents and then eliminating senescent cancer cells by directly targeting them. However, this would require the development of biomarkers to classify cells exhibiting TIS as either tumour-suppressive or pro-tumorigenic90.
Interestingly, an elegant inducible genetic system to eliminate in mice the p16INK4a-positive, senescent cells demonstrated that such elimination of senescent cells delayed aged-related disorders80,136. Thus, therapeutic elimination of senescent cells could be a good approach to delay and/or to treat age-related diseases, including the pro-tumorigenic effects of senescent cells.
Another therapeutic strategy that has been considered is to promote homogeneous senescence within the tumour. A brief exposure to Palbociclib via lysosomal trapping selectively inhibits CDK4/6, resulting in stable cell-cycle arrest and long-term senescence137. Moreover, based on CRISPR-mediated genetic and chemical screens, it was proposed that suppressing the SWI/SNF component SMARCB1 induces senescence in melanoma by strongly activating the MAP kinase pathway138. However, inducing homogenous senescence could be challenging, and senescent cells could rewire the tumour microenvironment in ways that would promote tumour relapse, potentially making this strategy more harmful than helpful in some cases. Furthermore, a small fraction of senescent cells could escape from their dormant state by senescence-associated stemness, and hence promote tumour growth potential30. Therefore, pharmacological strategies aimed to eliminate senescent cells before a fraction of them implement features of senescence-associated stemness and re-enter cell cycle would certainly avoid tumour relapse.
Concluding remarks
Tumour relapse is a complex and poorly defined phenomenon that limits our ability to completely cure cancer. Several studies have highlighted the presence of slow-cycling or slow-proliferating cancer cells and senescent cancer cell populations in tumours, neither of which are targeted by common cancer treatments, such as chemo and radiotherapy. These persistent cancer cell population is residual and undetectable and might be the cells at the origin of tumour relapse after several months or even years and once the treatment is stopped. Likewise, TIS could also induce dormancy through the appearance of senescent cells. Quiescent cells are supposed to contribute to tumour relapse by re-entering the cell cycle most likely due to appropriate fine-tuned microenvironment, while senescent cells may reinforce tumour regrowth though SASP and immune system modulation. The crosstalk between slow-cycling and senescent cells is not excluded and SASP secretion could fuel the proliferation of slow-cycling cells. SASP could also induce stemness of surrounding cells arguing to the pro- and anti-tumorigenic roles of senescent cells. However, much remains to be learned about the exact role of dormant cells in tumour recurrence. To this end, there is a clear need for new biomarkers and genetically engineered mouse models that can be used to label, track and monitor dormant cells after chemo- or radiotherapy to clarify their roles and functions during tumour relapse. This in turn may facilitate the design of new drugs targeting dormant cells and guide the development of new therapies to prevent the potentially fatal recurrence of tumours in cancer survivors.
Supplementary information
Acknowledgements
This work was funded by the State Research Agency (AEI, 10.13039/501100011033) from the Spanish Ministry of Science and Innovation (projects granted to N.D. SAF2016-76598-R, SAF2017-92733-EXP, RTI2018-094834-B-I00 and RED2018-102723-T), cofounded by European Regional Development Fund (ERDF). K.S.D.F. is recipient of a fellowship from the AECC Scientific Foundation (Madrid). This work was developed at the CNIO funded by the Health Institute Carlos III (ISCIII) and the Spanish Ministry of Science and Innovation.
Author contributions
K.S.D.F. and N.D. wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Christina Karlsson Rosenthal. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-021-02257-0.
References
- 1.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puig I, et al. TET2 controls chemoresisstant slow-cycling cancer cell survival and tumor recurrence. J. Clin. Investig. 2018;128:3887–3905. doi: 10.1172/JCI96393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, et al. A restricted cell population propagates glioblastoma growth following chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal P, et al. Mesenchymal niche-specific expression of Cxcl12 controls quiescence of treatment-resistant leukemia stem cells. Cell Stem Cell. 2019;24:769–784. doi: 10.1016/j.stem.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dembinski JL, Krauss S. Characterization and functional analysis of a slow cycling stem cell-like subpopulation in pancreas adenocarcinoma. Clin. Exp. Metastas-. 2009;26:611–623. doi: 10.1007/s10585-009-9260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin W-c, et al. Dormant cancer cells contribute to residual disease in a model of reversible pancreatic cancer. Cancer Res. 2013;73:1821–1830. doi: 10.1158/0008-5472.CAN-12-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole AJ, et al. NFATC4 promotes quiescence and chemotherapy resistance in ovarian cancer. JCI Insight. 2020;5:e131486. doi: 10.1172/jci.insight.131486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolter K, Zender L. Therapy-induced senescence — an induced synthetic lethality in liver cancer. Nat. Rev. Gastro Hepat. 2019;17:135–136. doi: 10.1038/s41575-020-0262-3. [DOI] [PubMed] [Google Scholar]
- 9.Clarke MF, et al. Cancer stem cells-perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 10.Aguirre‑Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat. Rev. Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agudo J, et al. Quiescent tissue stem cells evade immune surveillance. Immunity. 2018;20:271–285.e275. doi: 10.1016/j.immuni.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchison TJ. The proliferation rate paradox in antimitotic chemotherapy. Mol. Biol. Cell. 2012;23:1–6. doi: 10.1091/mbc.e10-04-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spinella J-F, et al. Mutational dynamics of early and late relapsed childhood ALL: rapid clonal expansion and long-term dormancy. Blood Adv. 2018;2:177–188. doi: 10.1182/bloodadvances.2017011510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen K, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children’s Oncology Group study. Leukemia. 2008;22:2142–2150. doi: 10.1038/leu.2008.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeshita T, Yan L, Asaoka M, Rashid O, takabe K. Late recurrence of breast cancer is associated with pro-cancerous immune microenvironment in the primary tumor. Sci. Rep. 2019;15:16942. doi: 10.1038/s41598-019-53482-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayat, M. A. in Tumor Dormancy, Quiescence and Senescence, Vol. 1. Aging, cancer and noncancer pathologies (ed. M. A. Hayat) (Springer, 2013).
- 18.Zhang, X. H. F., Giuliano, M., Trivedi, M. V., Schiff, R. & Osborne, C. K. Metastasis dormancy in estrogen receptor-positive breast cancer. Clin. Cancer Res. 19, 6389–6397 (2013). [DOI] [PMC free article] [PubMed]
- 19.Liver, E. A. F. T. S. O. T. EASL clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 69, 182–236 (2018). [DOI] [PubMed]
- 20.Thakkar JP, et al. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014;23:1985–1996. doi: 10.1158/1055-9965.EPI-14-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sánchez-Danés A, et al. A slow-cycling LGR5 tumour population mediates basal cell carcinoma relapse after therapy. Nature. 2018;562:434–438. doi: 10.1038/s41586-018-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bao S, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 23.Hong SP, et al. Single-cell transcriptomics reveals multi-step adaptations to endocrine therapy. Nat. Commun. 2019;10:3840. doi: 10.1038/s41467-019-11721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rehman SK, et al. Colorectal cancer cells enter a diapause-like DTP state to survive chemotherapy. Cell. 2021;184:226–242. doi: 10.1016/j.cell.2020.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhimolea E, et al. An embryonic diapause-like adaptation with suppressed myc activity enables tumor treatment persistence. Cancer Cell. 2021;39:240–256. doi: 10.1016/j.ccell.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreso A, et al. Variable clonal repopulation dynamics influence chemotherapy response in colorectal cancer. Science. 2013;339:543–548. doi: 10.1126/science.1227670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Triana-Martínez, F., Loza, M. I. & Domínguez, E. Beyond tumor suppression: senescence in cancer stemness and tumor dormancy. Cells9, 10.3390/cells9020346 (2020). [DOI] [PMC free article] [PubMed]
- 28.Rao SG, Jackson JG. SASP: tumor suppressor or promoter? Yes! Trends. Cancer. 2016;2:676–687. doi: 10.1016/j.trecan.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat. Rev. Genet. 2008;9:115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- 30.Milanovic M, et al. Senescence-associated reprogramming promotes cancer stemness. Nature. 2018;553:96–100. doi: 10.1038/nature25167. [DOI] [PubMed] [Google Scholar]
- 31.Demaria M, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angelis MLD, Francescangeli F, Torre FL, Zeuner A. Stem cell plasticity and dormancy in the development of cancer therapy resistance. Front Oncol. 2019;9:1–14. doi: 10.3389/fonc.2019.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Besson A, et al. A pathway in quiescent cells that controls p27Kip1 stability, subcellular localization, and tumor suppression. Genes Dev. 2006;20:47–64. doi: 10.1101/gad.1384406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodgers JT, et al. mTORC1 controls the adaptive transitionofquiescent stem cells from G0 to GAlert. Nature. 2014;510:393–396. doi: 10.1038/nature13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valcourt JR, et al. Staying alive: metabolic adaptations to quiescence. Cell Cycle. 2012;11:1680–1696. doi: 10.4161/cc.19879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beerman I, Seita J, Inlay MA, Weissman IL, Rossi DJ. Quiescent hematopoietic stem cells accumulate DNA damage during aging that is repaired upon entry into cell cycle. Cell Stem Cell. 2014;15:37–50. doi: 10.1016/j.stem.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohrin M, et al. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell. 2010;7:174–185. doi: 10.1016/j.stem.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y, et al. Single-cell landscape of the ecosystem in earlyrelapse hepatocellular carcinoma. Cell. 2021;184:404–421.e416. doi: 10.1016/j.cell.2020.11.041. [DOI] [PubMed] [Google Scholar]
- 39.Iannello A, Thompson TW, Ardolino M, Lowe SW, Raulet DH. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J. Exp. Med. 2013;210:2057–2069. doi: 10.1084/jem.20130783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cosman D, et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/S1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 41.Jamieson AM, et al. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. doi: 10.1016/S1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 42.Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev. Immunol. 2013;31:413–441. doi: 10.1146/annurev-immunol-032712-095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malladi, S. et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell165, 10.1016/j.cell.2016.02.025 (2016). [DOI] [PMC free article] [PubMed]
- 44.Biehs B, et al. A cell identity switch allows residual BCC to survive Hedgehog pathway inhibition. Nature. 2018;562:429–433. doi: 10.1038/s41586-018-0596-y. [DOI] [PubMed] [Google Scholar]
- 45.Brown JA, et al. TGF-β-induced quiescence mediates chemoresistance of tumor-propagating cells in squamous cell carcinoma. Cell Stem Cell. 2017;21:650–664. doi: 10.1016/j.stem.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharpless NE, Sherr CJ. Forging a signature of in vivo senescence. Nat. Rev. Cancer. 2015;15:397–408. doi: 10.1038/nrc3960. [DOI] [PubMed] [Google Scholar]
- 47.Stein GH, Dulić V. Origins of G1 arrest in senescent human fibroblasts. Bioessays. 1995;17:537–543. doi: 10.1002/bies.950170610. [DOI] [PubMed] [Google Scholar]
- 48.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 49.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/S0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 50.Beausejour CM, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campisi J, Fagagna fDAD. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 52.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dulić V, et al. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;76:1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 54.Serrano M. The tumor suppressor protein p16INK4a. Exp. Cell Res. 1997;237:7–13. doi: 10.1006/excr.1997.3824. [DOI] [PubMed] [Google Scholar]
- 55.Narita M, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/S0092-8674(03)00401-X. [DOI] [PubMed] [Google Scholar]
- 56.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee BY, et al. Senescence-associated β -galactosidase is lysosomal β-galactosidase. Aging Cell. 2006;5:187–195. doi: 10.1111/j.1474-9726.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- 58.Dimri GP, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. P Natl Acad. Sci. USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kobayashi A, et al. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J. Exp. Med. 2011;208:2641–2655. doi: 10.1084/jem.20110840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma S, et al. Secreted Protein Acidic and Rich in Cysteine (SPARC) mediates metastatic dormancy of prostate cancer in bone. J. Biol. Chem. 2016;291:19351–19363. doi: 10.1074/jbc.M116.737379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bulavin DV, Fornace AJ. p38 MAP kinase’s emerging role as a tumor suppressor. Adv. Cancer Res. 2004;92:95–118. doi: 10.1016/S0065-230X(04)92005-2. [DOI] [PubMed] [Google Scholar]
- 62.Roberson RS, et al. Escape from therapy-induced accelerated cellular senescence in p53-null lung cancer cells and in human lung cancers. Cancer Res. 2005;65:2795–2803. doi: 10.1158/0008-5472.CAN-04-1270. [DOI] [PubMed] [Google Scholar]
- 63.Takahashi A, et al. Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nat. Cell Biol. 2006;8:1291–1297. doi: 10.1038/ncb1491. [DOI] [PubMed] [Google Scholar]
- 64.Frey N, Venturelli S, Zender L, Bitzer M. Cellular senescence in gastrointestinal diseases: from pathogenesis to therapeutics. Nat. Rev. Gastro Hepat. 2017;15:81–95. doi: 10.1038/nrgastro.2017.146. [DOI] [PubMed] [Google Scholar]
- 65.Lee S, Schmitt CA. The dynamic nature of senescence in cancer. Nat. Cell Biol. 2019;21:94–101. doi: 10.1038/s41556-018-0249-2. [DOI] [PubMed] [Google Scholar]
- 66.Pérez‑Mancera PA, Young ARJ, Narita M. Inside and out: the activities of senescence in cancer. Nat. Rev. Cancer. 2014;14:547–558. doi: 10.1038/nrc3773. [DOI] [PubMed] [Google Scholar]
- 67.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 68.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karin M. Nuclear factor-κB in cancer development and progressio. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 70.Eggert T, et al. Distinct functions of senescence-associated immune responses in liver tumor surveillance and tumor progression. Cancer cell. 2016;30:533–547. doi: 10.1016/j.ccell.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ritschka, B. et al. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev31, 10.1101/gad.290635.116 (2017). [DOI] [PMC free article] [PubMed]
- 72.Mosteiro L, et al. Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science. 2016;354:aaf4445. doi: 10.1126/science.aaf4445. [DOI] [PubMed] [Google Scholar]
- 73.Cahu, J., Bustany, S. & Sola, B. Senescence-associated secretory phenotype favors the emergence of cancer stem-like cells. Cell Death Dis. 3, 10.1038/cddis.2012.183 (2012). [DOI] [PMC free article] [PubMed]
- 74.Canino C, et al. SASP mediates chemoresistance and tumor-initiating-activity of mesothelioma cells. Oncogene. 2012;31:3148–3163. doi: 10.1038/onc.2011.485. [DOI] [PubMed] [Google Scholar]
- 75.Muñoz-Espín D, et al. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155:1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 76.Storer M, et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155:1119–1130. doi: 10.1016/j.cell.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 77.Helman A, et al. p16Ink4a-induced senescence of pancreatic beta cells enhances insulin secretion. Nat. Med. 2016;22:412–420. doi: 10.1038/nm.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He S, Sharpless NE. Senescence in health and disease. Cell. 2017;169:1000–1011. doi: 10.1016/j.cell.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 80.Baker DJ, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baar MP, et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell. 2017;169:132–147. doi: 10.1016/j.cell.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Childs BG, et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016;354:472–477. doi: 10.1126/science.aaf6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wiemann SU, et al. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 2002;16:935–942. doi: 10.1096/fj.01-0977com. [DOI] [PubMed] [Google Scholar]
- 84.Krizhanovsky V, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang B-D, et al. A senescence-like phenotype distinguishes tumor cells that undergo terminal proliferation arrest after exposure to anticancer agents. Cancer Res. 1999;59:3761–3767. [PubMed] [Google Scholar]
- 86.Kovatcheva M, et al. ATRX is a regulator of therapy induced senescence in human cells. Nat. Commun. 2017;8:386. doi: 10.1038/s41467-017-00540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bromfield G, Meng A, Warde P, Bristow R. Cell death in irradiated prostate epithelial cells: role of apoptotic and clonogenic cell kill. Prostate Cancer Prostatic Dis. 2003;6:73–85. doi: 10.1038/sj.pcan.4500628. [DOI] [PubMed] [Google Scholar]
- 88.Rodier F, et al. Persistent DNA damage signalling triggers senescenceassociated inflammatory cytokine secretion. Nat. Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tsai KKC, Stuart J, Chuang Y-YE, Little JB, Yuan Z-M. Low-dose radiation-induced senescent stromal fibroblasts render nearby breast cancer cells radioresistant. Radiat. Res. 2009;172:306–313. doi: 10.1667/RR1764.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murray, D. & Mirzayans, R. Role of therapy-induced cellular senescence in tumor cells and its modification in radiotherapy: the good, the bad and the ugly. J Nucl Med Radiat Ther. S6, 10.4172/2155-9619.S6-018 (2013).
- 91.Poele RHT, Okorokov AL, Jardine L, Cummings J, Joel SP. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 2002;62:1876–1883. [PubMed] [Google Scholar]
- 92.Hoare M, et al. NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat. Cell Biol. 2016;18:979–992. doi: 10.1038/ncb3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ito Y, Hoare M, Narita M. Spatial and temporal control of senescence. Trends Cell Biol. 2017;27:820–832. doi: 10.1016/j.tcb.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 94.Xue W, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kang T-W, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 96.Langhi Prata, L. G. P., Ovsyannikova, I. G., Tchkonia, T. & Kirkland, J. L. Senescent cell clearance by the immune system: emerging therapeutic opportunities. Semin Immunol.40, 10.1016/j.smim.2019.04.003 (2018). [DOI] [PMC free article] [PubMed]
- 97.Reimann, M. et al. Adaptive T-cell immunity controls senescence-prone MyD88- or CARD11-mutant B-cell lymphomas. Blood 10.1182/blood.2020005244. (2020). [DOI] [PubMed]
- 98.Ruscetti M, et al. NK cell–mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science. 2018;362:1416–1422. doi: 10.1126/science.aas9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ruscetti M, et al. Senescence-induced vascular remodeling creates therapeutic vulnerabilities in pancreas cancer. Cell. 2020;181:424–441. doi: 10.1016/j.cell.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Krtolica A, Parrinello S, Lockett S, Desprez P-Y, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: A link between cancer and aging. P Natl Acad. Sci. Usa. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang C, et al. Inducing and exploiting vulnerabilities for the treatment of liver cancer. Nature. 2019;547:268–272. doi: 10.1038/s41586-019-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Essers MAG, et al. IFNα activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 103.Saito Y, et al. Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AMl. Nat. Biotechnol. 2010;28:275–280. doi: 10.1038/nbt.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Krug U, et al. Increasing intensity of therapies assigned at diagnosis does not improve survival of adults with acute myeloid leukemia. Leukemia. 2016;30:1230–1236. doi: 10.1038/leu.2016.25. [DOI] [PubMed] [Google Scholar]
- 105.Chaves-Pérez, A., Yilmaz, M., Perna, C., Rosa, S. D. L. & Djouder, N. URI is required to maintain intestinal architecture during ionizing radiation. Science364, 10.1126/science.aaq1165 (2019). [DOI] [PubMed]
- 106.Bragado P, et al. TGFβ2 dictates disseminated tumour cell fate in target organs through TGFβ-RIII and p38α/β signalling. Nat. Cell Biol. 2013;15:1351–1361. doi: 10.1038/ncb2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Park SY, Nam JS. The force awakens: metastatic dormant cancer cells. Exp. Mol. Med. 2020 doi: 10.1038/s12276-020-0423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aguirre-Ghiso JA, Estrada Y, Liu D, Ossowski L. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK) Cancer Res. 2003;63:1684–1695. [PubMed] [Google Scholar]
- 110.Aguirre-Ghiso JA, Liu D, Mignatti A, Kovalski K, Ossowski L. Urokinase receptor and fibronectin regulate the ERKMAPK to p38MAPK activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol. Biol. Cell. 2001;12:863–879. doi: 10.1091/mbc.12.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Touny LHE, et al. Combined SFK/MEK inhibition prevents metastatic outgrowth of dormant tumor cells. J. Clin. Investig. 2014;124:156–168. doi: 10.1172/JCI70259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barkan D, et al. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. 2010;70:5706–5716. doi: 10.1158/0008-5472.CAN-09-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barkan D, et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 2008;68:6241–6250. doi: 10.1158/0008-5472.CAN-07-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Adam AP, et al. Computational identification of a p38SAPK regulated transcription factor network required for tumor cell quiescence. Cancer Res. 2009;69:5664–5672. doi: 10.1158/0008-5472.CAN-08-3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Salvador-Barbero, B. et al. CDK4/6 inhibitors impair recovery from cytotoxic chemotherapy in pancreatic adenocarcinoma. Cancer cell37, 10.1016/j.ccell.2020.01.007 (2020). [DOI] [PubMed]
- 116.Rajbhandari N, Lin W-c, Wehde BL, Triplett AA, Wagner K-U. Autocrine IGF1 signaling mediates pancreatic tumor cell dormancy in the absence of oncogenic drivers. Cell Rep. 2017;18:2243–2255. doi: 10.1016/j.celrep.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zeuner A, et al. Elimination of quiescent/slow-proliferating cancer stem cells by Bcl-XL inhibition in non-small cell lung cancer. Cell Death Differ. 2014;21:1877–1888. doi: 10.1038/cdd.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Viswanathan VS, et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547:453–457. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hangauer MJ, et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551:247–250. doi: 10.1038/nature24297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roesch A, et al. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141:583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Amor C, et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature. 2020;583:127–132. doi: 10.1038/s41586-020-2403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Naylor R, Baker D, Deursen JV. Senescent cells: a novel therapeutic target for aging and age-related diseases. Clin. Pharm. Ther. 2013;93:105–116. doi: 10.1038/clpt.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Toso A, et al. Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep. 2014;9:75–89. doi: 10.1016/j.celrep.2014.08.044. [DOI] [PubMed] [Google Scholar]
- 124.Laberge R-M, et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 2015;17:1049–1061. doi: 10.1038/ncb3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brandt M, et al. mTORC1 inactivation promotes colitis-induced colorectal cancer but protects from APC loss-dependent tumorigenesis. Cell Metab. 2018;27:118–135. doi: 10.1016/j.cmet.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 126.Umemura A, et al. Liver damage, inflammation, and enhanced tumorigenesis after persistent mTORC1 inhibition. Cell Metab. 2014;20:133–144. doi: 10.1016/j.cmet.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Omer A, et al. G3BP1 controls the senescence-associated secretome and its impact on cancer progression. Nat. Commun. 2020;11:4979. doi: 10.1038/s41467-020-18734-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bird, T. G. et al. TGFβ inhibition restores a regenerative response in acute liver injury by suppressing paracrine senescence. Sci Transl Med.10, 10.1126/scitranslmed.aan1230 (2018). [DOI] [PMC free article] [PubMed]
- 129.Zhu Y, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Serrano M, Barzilai N. Targeting senescence. Nat. Med. 2018;24:1089–1096. doi: 10.1038/s41591-018-0141-4. [DOI] [PubMed] [Google Scholar]
- 131.Muñoz-Espín, D. et al. A versatile drug delivery system targeting senescent cells. EMBO Mol Med. 9, 10.15252/emmm.201809355 (2018). [DOI] [PMC free article] [PubMed]
- 132.Chang J, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kurppa KJ, et al. Treatment-induced tumor dormancy through YAP-mediated transcriptional reprogramming of the apoptotic pathway. Cancer Cell. 2020;37:104–122. doi: 10.1016/j.ccell.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sieben CJ, Sturmlechner I, Sluis BVD, Deursen JMV. Two-step senescence-focused cancer therapies. Trends Cell Biol. 2018;28:723–737. doi: 10.1016/j.tcb.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pajvani UB, et al. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat. Med. 2005;11:797–803. doi: 10.1038/nm1262. [DOI] [PubMed] [Google Scholar]
- 137.Llanos S, Megias D, Pietrocola F, Blanco-Aparicio C, Serrano M. Lysosomal trapping of palbociclib and its functional implications. Oncogene. 2019;38:3886–3902. doi: 10.1038/s41388-019-0695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang L, et al. High-throughput functional genetic and compound screens identify targets for senescence induction in cancer. Cell Rep. 2017;21:773–783. doi: 10.1016/j.celrep.2017.09.085. [DOI] [PubMed] [Google Scholar]
- 139.Pardee AB. G1 events and regulation of cell proliferation. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- 140.Evertts AG, et al. H4K20 methylation regulates quiescence and chromatin compaction. Mol. Biol. Cell. 2013;24:3025–3037. doi: 10.1091/mbc.e12-07-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.