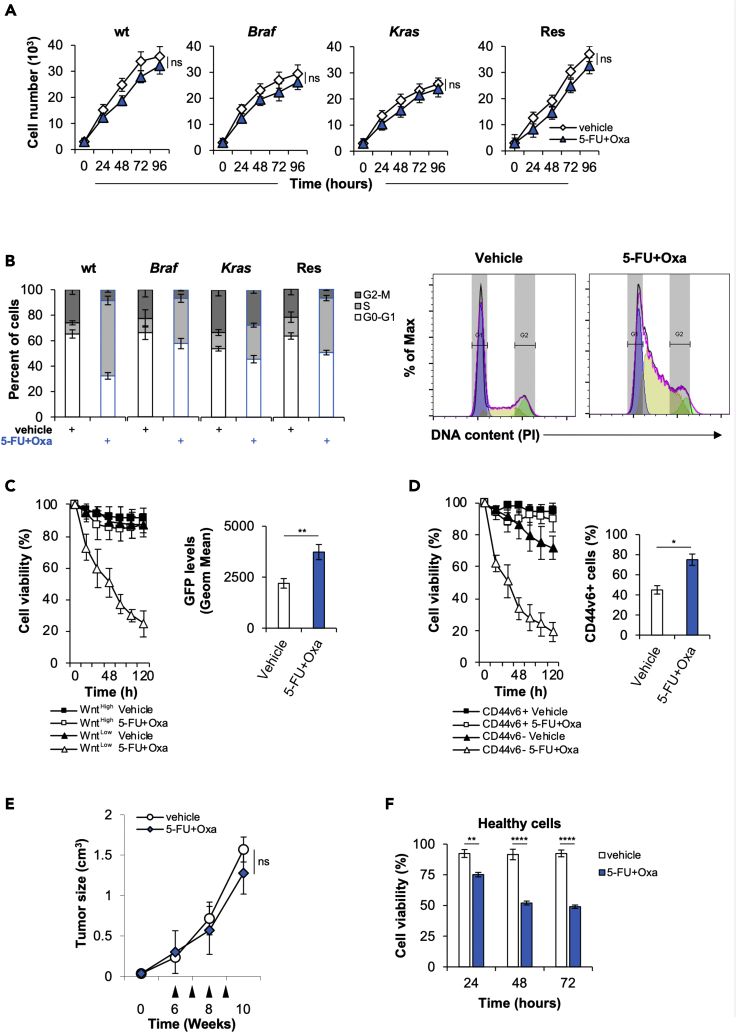
Figure 1.
CR-CSphCs are endowed with innate chemoresistance
(A) Cell growth kinetics of CR-CSphCs treated with a vehicle or 5-FU in combination with oxaliplatin, up to 4 days. Data represent the mean ± SD (n = 3) using 2 different CR-CSphC lines for each subgroup (wt, #7 and #21; Braf, #3 and #5; Kras #8 and #9; Res #R3 and #R4). Statistical significance between 2 groups was determined by unpaired Student's t-test (2-tailed). ns, nonsignificant.
(B) Cell cycle analysis in CR-CSphCs treated as in (A) for 24 h. Data show percentage of cell number in G0–G1, S, and G2–M phases. Data are expressed as mean ± SD of three independent experiments performed in CR-CSphCs isolated from patients with wt (#7 and #21), Braf (#3 and #5), Kras (#8 and #9) and chemoresistant (#R3 and #R4) CRC. (Right panels) Representative cell cycle analysis of CR-CSphCs treated with a vehicle or 5-FU in combination with oxaliplatin, for 24 h (blue color = G0-G1; yellow color = S; green color = G2-M).
(C) Percentage of viability in flow-cytometry-sorted TOP-GFP CR-CSphCs, treated as in (A) up to 120 h. Data are expressed as mean ± SD of three independent experiments performed using two different CR-CSphC lines (#8, #9). (Right panel) Representative flow cytometry analysis of TOP-GFP expression in spared CR-CSphCs, at 5 days. Statistical significance between 2 groups was determined by unpaired Student's t-test (2-tailed). ∗∗p ≤ 0.01.
(D) Cell viability percentage of CR-CSphCs enriched for CD44v6 expression and treated with vehicle or 5-FU in combination with oxaliplatin up to 120 h. Data are expressed as mean ± SD of three independent experiments performed using four different CR-CSphCs lines (#3, #9, #21, #R4). (Right panel) Representative flow cytometry analysis of the percentage of CD44v6 positivity in spared CR-CSphCs, at 5 days. Statistical significance between 2 groups was determined by unpaired Student's t-test (2-tailed). ∗p ≤ 0.05.
(E) Tumor size of CR-CSphCs subcutaneously injected into immunocompromized mice, treated for 4 weeks (from sixth to ninth week) with vehicle or 5-FU in combination with oxaliplatin. Data represent the mean ± SD of tumor size measured in six mice per group, using 2 different CR-CSphC lines (#8, #21). Black arrowheads indicate weeks of treatment. Statistical significance between 2 groups was determined by unpaired Student's t-test (2-tailed). ns, nonsignificant.
(F) Cell viability analysis of healthy cells (IMEC and AD-MSCs) treated as in (D), for 3 days. Data are expressed as mean ± SD of three independent experiments. Statistical significance between 2 groups was determined by unpaired Student's t-test (2-tailed).∗∗p ≤ 0.01; ∗∗∗∗p ≤ 0.0001. See also Figure S1.