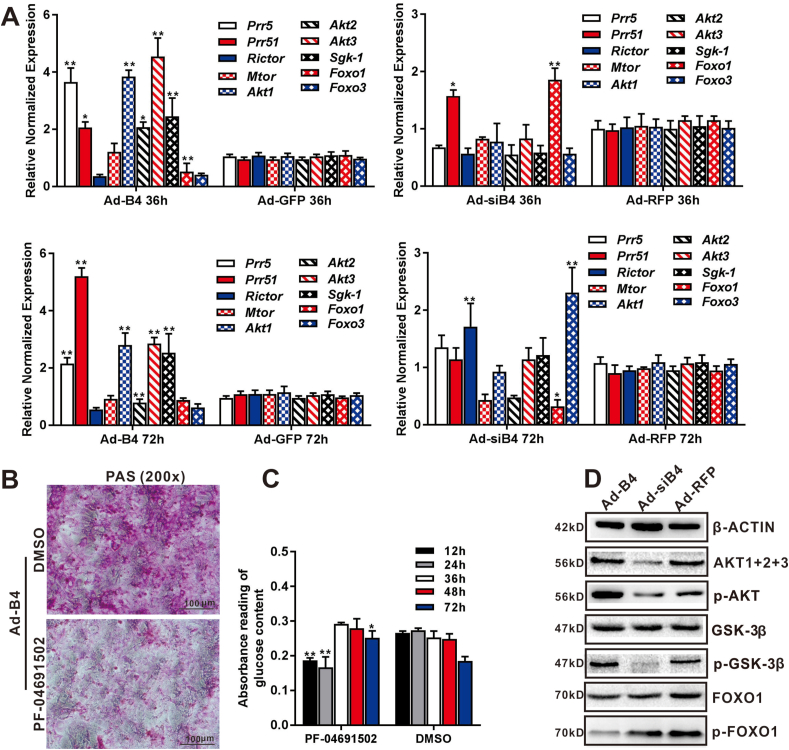
Figure 3.
BMP4 regulates hepatic glucose metabolism through mTORC2 signaling. Subconfluent MPH cells were infected with Ad-B4, Ad-GFP, Ad-siB4 and Ad-RFP for 36h and 72h. Total RNA was isolated and TqPCR analysis was carried out to detect the expression of essential members and downstream glucose metabolism related genes of mTORC2 signaling pathway at 36h and 72h, respectively. Relative expression was calculated by dividing the relative expression values (i.e., gene/Gapdh) in “∗∗” P < 0.01, “∗” P < 0.05, Ad-B4 group vs. Ad-GFP group, Ad-siB4 group vs. Ad-RFP group (A) Subconfluent MPH cells were infected with Ad-B4, treated with the 0.1 mM PI3K(α/β/δ/γ)/mTOR inhibitor PF-04691502 or DMSO vehicle control for 3 days, then subjected to PAS staining (magnification, x200) (B) Ad-B4 infected the MPH cells, and treated with PF-04691502 or DMSO for 12h, 24h, 36h, 48h and 72h. Total glucose levels in cells was assessed by absorbance reading at different time points. “∗∗” P < 0.01 PF-04691502 group vs. DMSO group (C) Subconfluent MPH cells were infected with Ad-B4, Ad-siB4 or Ad-RFP. Total cellular proteins were prepared and subjected to Western blotting to detect the expression or phosphorylation levels of genes related to glucose metabolism regulated by mTORC2 signaling pathway, including AKT1+2+3, p-AKT, GSK-3β, p-GSK-3β, FOXO1, p-FOXO1, while β-ACTIN was used as a loading control (D) Each assay condition was done in triplicate. Representative images are shown.