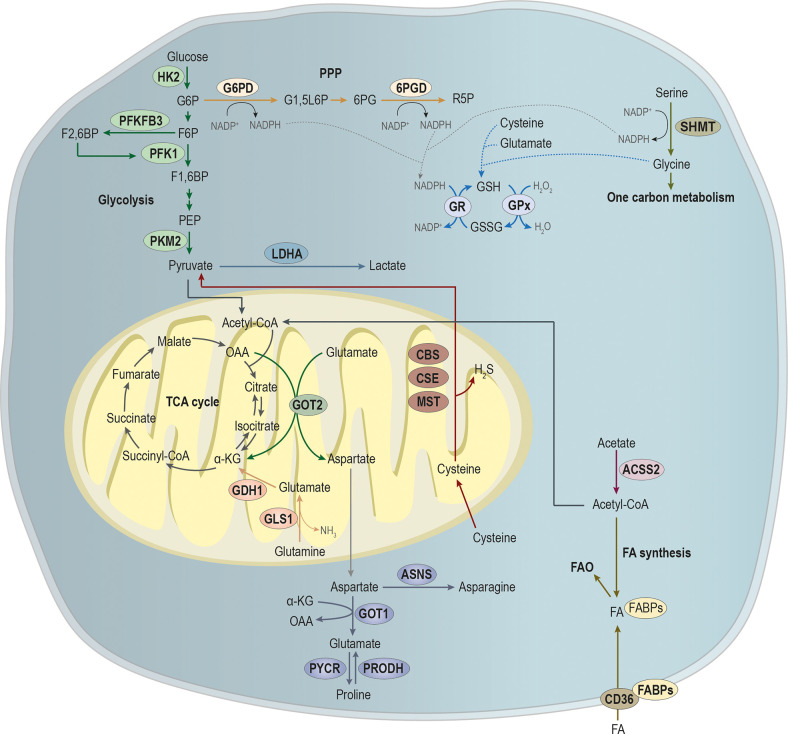
Figure 2.
Main pathways involved in the metabolic remodeling of metastatic cancer cells, pivotal for energy and biomass production. Metastatic cancer cells are metabolically more plastic than non-metastatic cancer cells, because they need to survive in primary tumor microenvironment, and they must also prosper in the microenvironment of the metastasized organ. Glycolysis is a multi-step process, in which the expression of key glycolytic enzymes as hexokinase 2 (HK2) that converts glucose in glucose-6-phosphate (G6P), 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) that promotes the conversion of fructose-6-phosphate (F6P) in fructose-2,6-biphosphate (F2,6BP; an allosteric activator of the glycolytic enzyme phosphofructokinase 1 (PFK1)) and pyruvate by tumor M2-pyruvate kinase (PKM2) that synthesizes pyruvate from phosphoenolpyruvic acid (PEP) have been showed to be involved in metastases establishment. Pyruvate is an endpoint product of glycolysis that after its conversion into acetyl-CoA, which can alternatively be produced from acetate, under the action of acyl-coenzyme A synthase short-chain family member 2 (ACSS2), then it will supply the tricarboxylic acid (TCA) cycle or the synthesis of fatty acids (FA). Additionality, FA can be imported by CD36, being the FA pool deviated to FA b-oxidation (FAO). Glycolysis intermediate glucose-6-phosphate (G6P) can be diverged from glycolysis to supply phosphate pentose pathway (PPP) through the action of glucose-6-phosphate dehydrogenase (G6PD), catalyzing the conversion of G6P into glucono-1,5-lactone-6P (G1,5L6P) and generating nicotinamide adenine dinucleotide phosphate (NADPH). The 6-phosphogluconate dehydrogenase (6PGD) converts 6-phosphogluconate (6PG) into ribose-5-phosphate (R5P), concomitantly with NAPDH production. Amino acids metabolism is crucial in biosynthesis and bioenergetics. The serine and glycine syntheses and the one-carbon metabolism play a central role in cell metabolism. The action of serine hydroxymethyltransferase (SHMT) catalyzes the conversion of serine into glycine, generating NADPH. NAPDH will be essential for the reduction of glutathione disulfide (GSSG) into glutathione (GSH) by the action of glutathione reductase (GR). In turn, the oxidation of GSH in GSSG by glutathione peroxidase (GPx) is essential for the control of the redox state of metastasizing cancer cells. Moreover, cysteine, glutamate and glycine are used in GSH synthesis. Glutamine catabolism through the action of glutaminase 1 (GLS1) leads to the production of glutamate and ammonia (NH3). In turn, glutamate dehydrogenase 1 (GLDH1) converts glutamate into α-ketoglutarate (α-KG) that will fulfill TCA cycle. In mitochondria, glutamate is a target of glutamic-oxaloacetic transaminase 2 (GOT-2), producing α-ketoglutarate (α-KG) and aspartate, at the expense of oxaloacetate (OAA). In cytoplasm, the action of GOT-1, at the expense α-KG, leads to glutamate production that can be converted into proline by the action of proline dehydrogenase (PRODH). Moreover, pyrroline-5-carboxylate reductase (PYCR) catalyzes the inverse reaction, leading to the conversion of proline into glutamate. Aspartate catabolism by asparagine synthetase (ASNS) promotes asparagine synthesis. Cysteine in cytoplasm is essential for GSH synthesis while in mitochondria, its catabolism by the cystathionine-β-synthase (CBS) route; the cystathionine-γ-lyase (CSE) route and the CAT/GOT:3-mercaptopyruvate sulfurtransferase (MST) axis route leads to the production of pyruvate and hydrogen sulfide (H2S).