Key Points
Question
Can a personalized topical cream formulated from coagulase-negative Staphylococcus with antimicrobial activity against Staphylococcus aureus from each specific individual with atopic dermatitis (AD) reduce S. aureus colonization and improve disease severity?
Findings
In this randomized, double-blind, placebo-controlled clinical trial of 11 patients with AD, S. aureus colonization was reduced by 99.2% in the treatment group compared with controls. Clinical improvement as assessed by local Eczema Area and Severity Index score after the end of treatment was also significantly improved compared with the vehicle.
Meaning
These findings suggest that a bacteriotherapy with an autologous strain of bacteria may safely improve S. aureus colonization in the microbiome of patients with AD and improve disease severity.
Abstract
Importance
Atopic dermatitis (AD) can be negatively affected by Staphylococcus aureus. The skin microbiome of AD is deficient in coagulase-negative Staphylococcus (CoNS) that can kill S aureus.
Objective
To evaluate if the antimicrobial-producing CoNS (CoNS-AM+) of a patient with AD can be autologously reintroduced to the same patient to inhibit survival of S aureus and improve clinical outcomes.
Design, Setting, and Participants
This double-blind, vehicle-controlled, single-center randomized clinical trial of 11 adult patients with moderate to severe AD who were randomized to receive either an autologous CoNS-AM+ (n = 5) or the vehicle (n = 6) was conducted between April 2016 and May 2018. The data were analyzed from May 2018 to July 2019.
Interventions
Autologous CoNS-AM+ was isolated from swabs that were obtained from the nonlesional skin of each patient with AD, expanded by culture, and then reapplied topically to the forearms at a concentration of 107 colony-forming units/g.
Main Outcomes and Measures
The primary end point of this study was to assess S aureus abundance after 1 week of application of autologous CoNS-AM+ on patients with AD by culture-based and DNA-based methods. The secondary end points were to assess the safety and clinical outcomes.
Results
Eleven patients (4 men [36.4%] and 7 women [63/6%]) were recruited based on the inclusion criteria. There were no serious adverse events in groups treated with autologous CoNS-AM+ or the vehicle. Staphylococcus aureus colonization on lesional skin at the end of treatment on patients who were treated with autologous CoNS-AM+ (mean of log10 ratio to baseline, −1.702; 95% CI, −2.882 to −0.523) was reduced by 99.2% compared with vehicle treatment (mean of log10 ratio to baseline, 0.671; 95% CI, −0.289 to 1.613; P = .01) and persisted for 4 days after treatment (CoNS-AM+: mean of log10 ratio to baseline, −1.752; 95% CI, −3.051 to −0.453; vehicle: mean of log10 ratio to baseline, −0.003; 95% CI, −1.083 to 1.076; P = .03). Importantly, local Eczema Area And Severity Index scores that were assessed at day 11 on patients who received CoNS-AM+ (mean of percentage change, −48.45; 95% CI, −84.34 to −12.55) were significantly improved compared with vehicle treatment (mean of percentage change, −4.52; 95% CI, −36.25 to 27.22; P = .04).
Conclusions and Relevance
The data from this randomized clinical trial suggest that bacteriotherapy with an autologous strain of skin commensal bacteria can safely decrease S aureus colonization and improve disease severity. Although larger studies will be needed, this personalized approach for S aureus reduction may provide an alternative treatment for patients with AD beyond antibiotics, immunosuppression, and immunomodulation.
Trial Registration
ClinicalTrials.gov Identifier: NCT03158012
This randomized clinical trial evaluates if the antimicrobial-producing coagulase-negative staphylococcus of a patient with atopic dermatitis can be autologously reintroduced to the same patient to inhibit survival of Staphylococcus aureus and improve clinical outcomes.
Introduction
A strong association between atopic dermatitis (AD) and Staphylococcus aureus (SA) colonization has been noted since 1974.1,2 The overgrowth of SA in AD has been attributed to diminished expression of human skin antimicrobial peptides, including cathelicidin and β-defensins, by direct action of TH2 cytokines, as well as defects in the skin barrier.3,4,5,6,7 Recently, we have found that an abnormality in the composition and function of the microbiome in patients with AD also contributes to SA overgrowth. Compared with individuals without AD, patients with AD lack coagulase-negative Staphylococcus (CoNS) that has the capacity to produce antimicrobials (AM) that inhibit SA (CoNS-AM+).8 In an effort to correct this defect, we previously demonstrated that a single application of autologous CoNS-AM+ reduced skin colonization by SA in AD skin.8 However, 1 application was not enough to show clinical improvement. Here, we hypothesize that extended treatment with autologous CoNS-AM+ for 1 week would be well tolerated and suppress SA survival in addition to promoting an improvement in each patient’s AD by correcting the defects in their skin microbiome.
Methods
This single-site, randomized, double-blind, vehicle-controlled trial was reviewed and approved by the US Food and Drug Administration as an investigational new drug application (IND# 15786), and is registered at ClinicalTrials.gov (NCT03158012) (Supplement 1). All experiments that involved human participants were conducted according to a protocol approved by the University of California, San Diego institutional review board. Written informed consent was obtained from all participants.
Coagulase-negative Staphylococcus AM+ was screened from the nonlesional skin of each patient with AD based on the ability to kill the SA strain that was isolated from lesional skin of the same patient. Subsequently, the CoNS-AM+ strain from each patient was formulated at 1 × 107 colony-forming units (CFUs) per gram in Cetaphil lotion (Galderma), which was confirmed not to affect bacterial viability. The vehicle cream was formulated with an equal amount of saline only. Two grams of the active lotion of CoNS-AM+ or the vehicle alone was applied to the ventral surface of the arm, including lesional (antecubital fossa) and nonlesional skin (forearm) sites. Treatment was performed on both arms twice a day for 7 days with the CoNS-AM+ matched to the patient from whom it was isolated. Skin swab results were obtained at day 0, day 4, day 7 (end of treatment), and days 8, 9, and 11 for follow-up points. Detailed methods are provided in the eMethods in Supplement 2. Analyses were conducted using Prism, version 8.0 (GraphPad), and statistical significance was set at P < .05.
Results
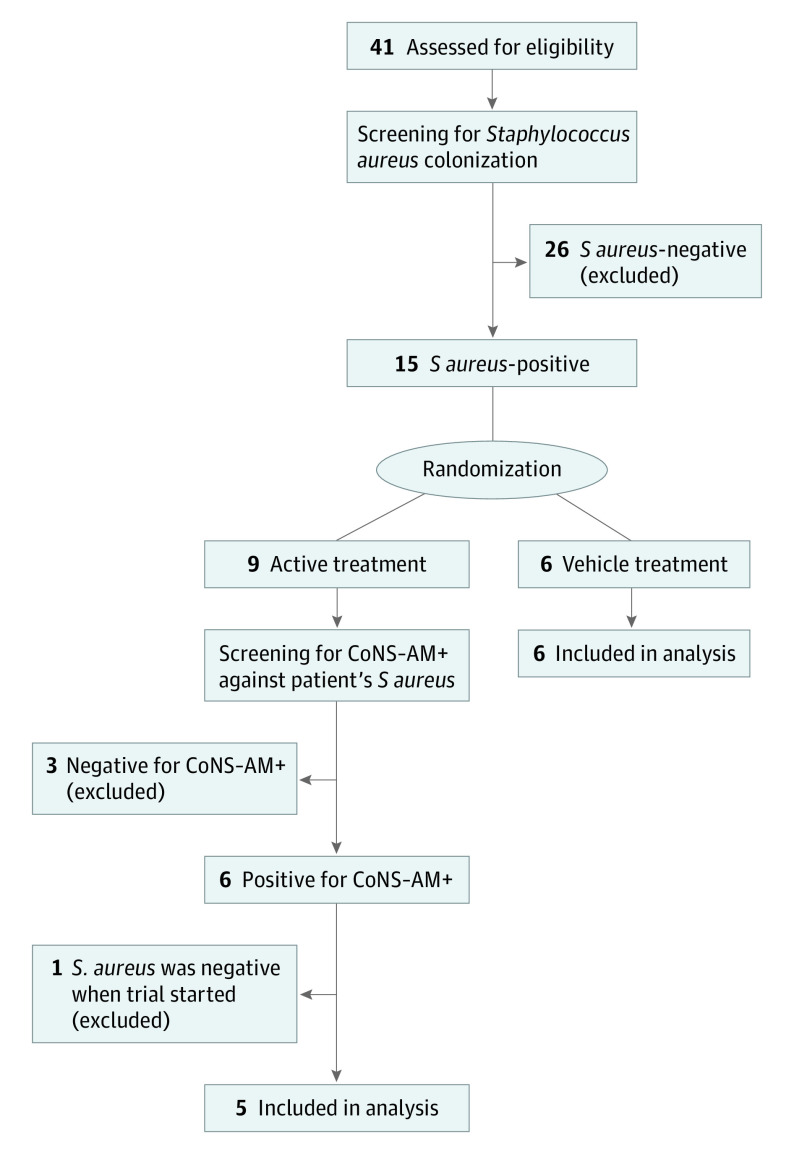
Forty-one patients with AD were screened for eligibility, and 15 were found to be culture-positive for SA. Nine of these patients were randomized to active treatment and 6 to the vehicle. Coagulase-negative staphylococcus bacteria were cultured from skin swab samples from nonlesional skin of each patient who was assigned to receive active treatment. Individual culture isolates were screened for the capacity to kill SA that was also isolated from the same participant (eFigure 1A in Supplement 2). Excluded from the study were 3 patients who failed to have CoNS-AM+ that were capable of inhibiting growth of their own, SA, and 1 patient who did not have SA at baseline (Figure 1). Baseline clinical characteristics were similar in the active and vehicle groups (eTable in Supplement 2).
Figure 1. CONSORT Flow Diagram of Participant Recruitment.
CoNS-AM+ indicates coagulase-negative Staphylococcus.
Species characterization of each individual CoNS-AM+ isolate revealed a variety of different strains with AM activity, including Staphylococcus epidermidis, Staphylococcus hominis, Staphylococcus warneri, and Staphylococcus capitis. These strains show potent AM activity against the SA strain that was isolated from the lesional skin of the same patient (eFigure 1B in Supplement 2).
There were no differences reported in the per-participant treatment-emergent adverse event rates, (0.40 for CoNS-AM+ and 0.33 for vehicle; mean risk ratio, 1.21; P > .99), and overall, only 2 mild adverse events were reported in each group, consisting of mild rash and stinging. Analysis of the absolute abundance of bacteria as measured by quantitative polymerase chain reaction for the 16S ribosomal RNA gene showed no difference on the lesional skin between the active- and vehicle-treated groups (Figure 2A). These data suggest that the continued reapplication of live CoNS-AM+ was well tolerated and did not lead to accumulation of bacteria on the skin surface.
Figure 2. One-Week Treatment With Autologous Antimicrobial-Producing Coagulase-Negative Staphylococcus (CoNS-AM+) Decreases Proportion of Staphylococcus aureus in the Microbial Community Without Leading to Excess Bacteria Accumulation on the Skin Surface.
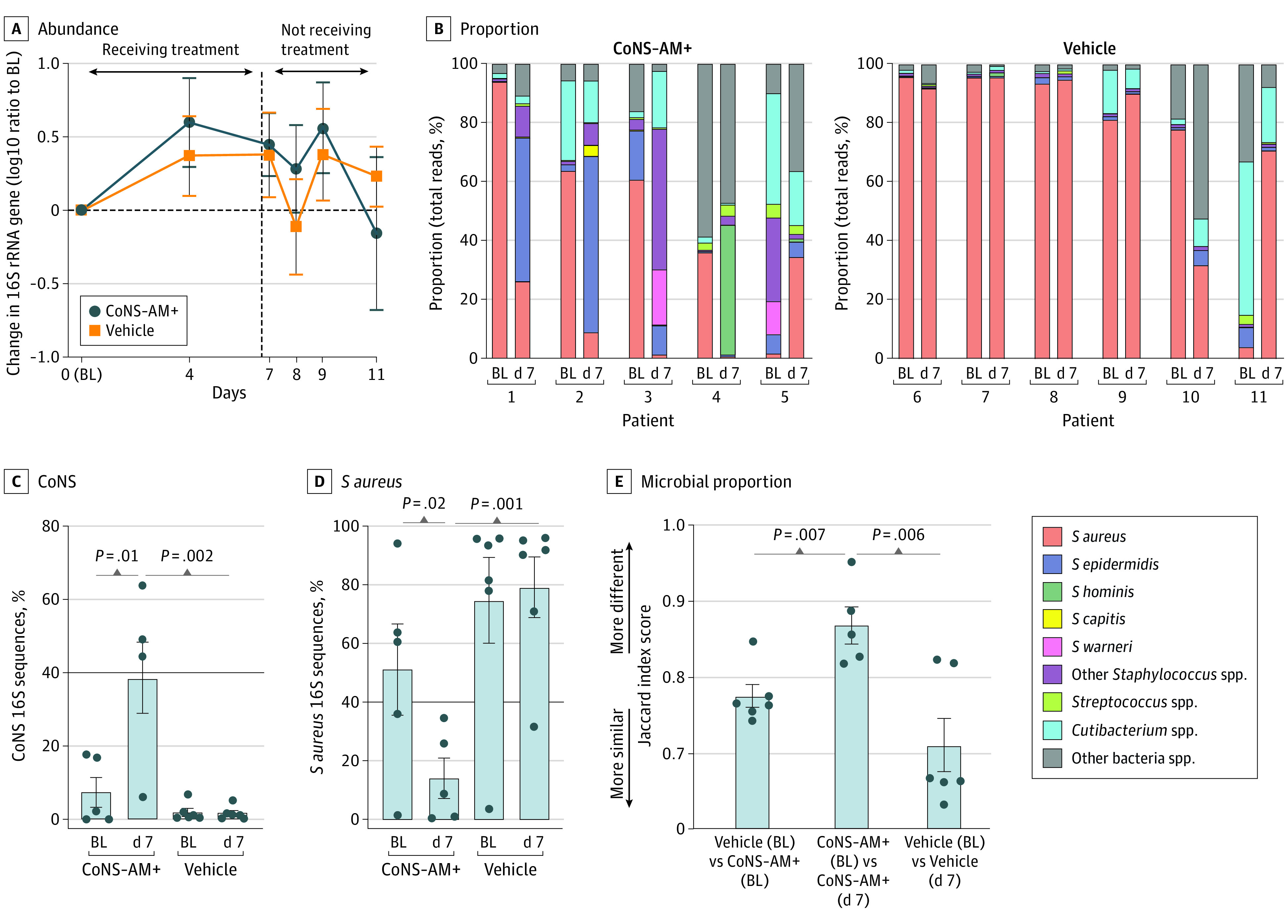
Effect of topical application of CoNS-AM+ or vehicle on the abundance (A) and proportion (B) of total 16S ribosomal RNA (rRNA) gene on the surface of lesional skin of patients with atopic dermatitis (AD). Data represent the mean (SEM) from each arm of individual patients. Data were compared with a 2-tailed parametric unpaired t test. The following CoNS-AM+ was transplanted to each patient: patient 1, Staphylococcus epidermidis-N009-G7; patient 2, Staphylococcus epiermidis-N018-F3; patient 3, Staphylococcus warneri-N025-G2; patient 4, Staphylococcus hominis-N005-E10; and patient 5, Staphylococcus capitis-N030-H8 (eFigure 1B in Supplement 2); spp indicates species. Relative abundance of CoNS (C) and S aureus 16S rRNA genes (D) determined from panel B. Data represent mean (SEM). Data were compared with a 2-tailed parametric paired (between points within the same group) or unpaired (between different treatment groups) t test. E, Dissimilarity in the microbial proportion calculated based on the Jaccard distance of the data from panel B. The P value was calculated by a 2-tailed parametric paired (baseline [BL] vs day 7 within the same patient’s group) or unpaired (BL data between different treatment group) t test.
Full-length 16S ribosomal RNA gene sequencing revealed an increase in the relative proportion of CoNS and a decrease in SA on lesional skin at the end of 1-week treatment with CoNS-AM+ compared with the baseline and the vehicle-treated group (Figure 2, B-D). The dissimilarities in the microbial proportion as assessed by Jaccard distance were found at the end of treatment with CoNS-AM+, but not with the vehicle (Figure 2E).
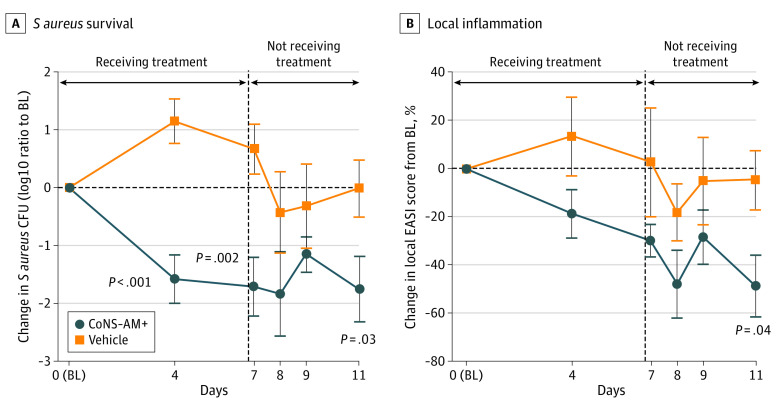
Staphylococcus aureus survival was also evaluated by live culture techniques. Application of CoNS-AM+ significantly decreased SA CFUs on lesional skin (Figure 3A). Notably, this suppression in live SA persisted even after the end of treatment for up to 4 days.
Figure 3. One-Week Treatment With Autologous Antimicrobial-Producing Coagulase-Negative Staphylococcus (CoNS-AM+) Inhibited Staphylococcus aureus Survival and Improved Local Disease Severity in Adult Patients With Atopic Dermatitis (AD).
Effect of twice-a-day topical application of CoNS-AM+ or vehicle on S aureus survival on the lesional skin of patients with AD (A) and local inflammation (B). S aureus survival was evaluated by counting colony forming units (CFUs) on an S aureus-selective agar plate. Data represent the mean (SEM) from each arm as the ratio to baseline (BL; day 0 point). Local inflammation was evaluated by calculating the local Eczema Area and Severity Index (EASI) score on the entire arm. Data represent mean (SEM). P values were calculated with a 2-tailed parametric unpaired t test.
Analysis of the local Eczema Area and Severity Index score on the ventral arms showed a decrease within 3 to 4 days of treatment, with statistical significance achieved when assessed 4 days after the end of the 1-week treatment period (Figure 3B). This suggested that application of the CoNS-AM+ not only decreases SA, but also improves AD in the treated area.
Discussion
This study demonstrates a suggested ability to improve an inherent defect in the skin microbiome of patients with AD by applying an autologous strain of bacteria with antimicrobial activity against SA. This study illustrated that skin commensal bacteria with beneficial actions can be therapeutic in AD and successfully inhibit SA.
Recently, our group conducted a randomized clinical trial of bacteriotherapy in 54 adult patients with AD using a universal defined CoNS-AM+ strain (S hominis A9) that was isolated from a healthy individual.9 This bacteriotherapy successfully inhibited SA survival and was associated with improvement in clinical AD in those patients with AD with SA that was sensitive to AM activity of S hominis A9. The sensitivity of SA strains to S hominis A9 seemed to correlate with early clinical improvement. In this trial, CoNS-AM+ from each individual was screened against the S aureus strain that was isolated from the same patient. This customized optimization of bacteriotherapy minimized the possibility of the existence of S aureus strains that are resistant to CoNS-AM+ and can improve the efficacy of treatment. Other open-label clinical trials with topical bacterial therapy have also suggested the potential for treatment of AD by a similar concept.10
Commensal CoNS may have the ability to improve AD though multiple therapeutic mechanisms. Some skin commensal CoNS inhibit toxin production by SA through blocking quorum sensing.11 In preclinical trials using various murine models, it has been demonstrated that the topical application of selected CoNS strains results in several beneficial actions to the host, including anti-inflammatory activity,12 promotion of wound healing,13,14 and cutaneous immune defense.15 These beneficial actions may be involved in additional mechanisms of action of bacteriotherapy in the improvement of AD.
Limitations
This study is limited by a single-center design and a small cohort. This clinical trial was also performed only on adults with SA-positive AD, which could select for a population with more inherently severe disease. Larger and longer duration clinical trials are needed to better assess the therapeutic potential of bacteriotherapy in all phenotypes of AD, including SA-culture negative and pediatric patients.
Conclusions
Early results from this study, as well as others, demonstrate promise for bacteriotherapy that utilizes protective skin commensal microbes in AD. Future investigations that target the microbiome in AD represent a therapeutic approach that may alter the pathogenesis of AD and explore its potential as an early intervention in pediatric AD.
Trial protocol
eFigure. Screening for autologous CoNS-AM+ with capacity to inhibit S. aureus
eTable. Clinical characteristics of patients with AD treated with CoNS-AM+ or the vehicle
eMethods.
Data sharing statement
References
- 1.Kong HH, Oh J, Deming C, et al. ; NISC Comparative Sequence Program . Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22(5):850-859. doi: 10.1101/gr.131029.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90(5):525-530. doi: 10.1111/j.1365-2133.1974.tb06447.x [DOI] [PubMed] [Google Scholar]
- 3.Mallbris L, Carlén L, Wei T, et al. Injury downregulates the expression of the human cathelicidin protein hCAP18/LL-37 in atopic dermatitis. Exp Dermatol. 2010;19(5):442-449. doi: 10.1111/j.1600-0625.2009.00918.x [DOI] [PubMed] [Google Scholar]
- 4.Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347(15):1151-1160. doi: 10.1056/NEJMoa021481 [DOI] [PubMed] [Google Scholar]
- 5.Hata TR, Kotol P, Boguniewicz M, et al. History of eczema herpeticum is associated with the inability to induce human β-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br J Dermatol. 2010;163(3):659-661. doi: 10.1111/j.1365-2133.2010.09892.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howell MD, Boguniewicz M, Pastore S, et al. Mechanism of HBD-3 deficiency in atopic dermatitis. Clin Immunol. 2006;121(3):332-338. doi: 10.1016/j.clim.2006.08.008 [DOI] [PubMed] [Google Scholar]
- 7.Howell MD, Gallo RL, Boguniewicz M, et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006;24(3):341-348. doi: 10.1016/j.immuni.2006.02.006 [DOI] [PubMed] [Google Scholar]
- 8.Nakatsuji T, Chen TH, Narala S, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med. 2017;9(378):eaah4680. doi: 10.1126/scitranslmed.aah4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat Med. 2021;27(4):700-709. doi: 10.1038/s41591-021-01256-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myles IA, Castillo CR, Barbian KD, et al. Therapeutic responses to Roseomonas mucosa in atopic dermatitis may involve lipid-mediated TNF-related epithelial repair. Sci Transl Med. 2020;12(560):eaaz8631. doi: 10.1126/scitranslmed.aaz8631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams MR, Costa SK, Zaramela LS, et al. Quorum sensing between bacterial species on the skin protects against epidermal injury in atopic dermatitis. Sci Transl Med. 2019;11(490):eaat8329. doi: 10.1126/scitranslmed.aat8329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scharschmidt TC, Vasquez KS, Pauli ML, et al. Commensal microbes and hair follicle morphogenesis coordinately drive treg migration into neonatal skin. Cell Host Microbe. 2017;21(4):467-477.e5. doi: 10.1016/j.chom.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linehan JL, Harrison OJ, Han SJ, et al. Non-classical immunity controls microbiota impact on skin immunity and tissue repair. Cell. 2018;172(4):784-796.e18. doi: 10.1016/j.cell.2017.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Constantinides MG, Link VM, Tamoutounour S, et al. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science. 2019;366(6464):eaax6624. doi: 10.1126/science.aax6624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naik S, Bouladoux N, Linehan JL, et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature. 2015;520(7545):104-108. doi: 10.1038/nature14052 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eFigure. Screening for autologous CoNS-AM+ with capacity to inhibit S. aureus
eTable. Clinical characteristics of patients with AD treated with CoNS-AM+ or the vehicle
eMethods.
Data sharing statement