FIGURE 1.
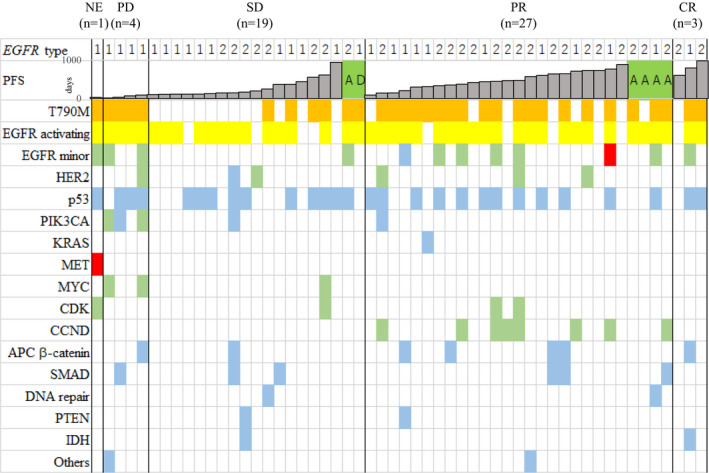
Molecular profile of circulating tumor DNA in plasma samples from patients prior to osimertinib treatment. Fifty‐four patients are shown among whom NGS was successful, from among 57 patients treated with osimertinib, including 3 CR, 27 PR, 19 SD, and 4 PD patients. One patient could not be evaluated for tumor response because of accidental development of cerebral infarction. Single‐nucleotide variants or insertions/deletions deemed to be oncogenic, likely oncogenic, or predicted oncogenic by OncoKB are included. NE, not evaluated; PD, progressive, disease; SD, stable disease; PR, partial response; CR, complete response; SNV/Indel, single‐nucleotide variants and/or insertion/deletions; CNV, copy number variants. In EGFR type, 1 and 2 indicate L858R and exon19 deletions, respectively.  , discontinuation by adverse effects;
, discontinuation by adverse effects;  , discontinuation at the request of the patient;
, discontinuation at the request of the patient;  , CNV;
, CNV;  , SNV/Indel;
, SNV/Indel;  , both CNV and SNV/Indel existed
, both CNV and SNV/Indel existed
